
Opioid free anesthesia for facial cosmetic surgery with Friedberg’s Triad
Friedberg’s Triad is a pathway to opioid free anesthesia (OFA) and better outcomes (1) (Figure 1).
Why measure the brain?
“If you cannot measure it, you cannot improve it.” —Lord Kelvin
Why preempt the pain?
“An ounce of prevention is worth a pound of cure.” —Benjamin Franklin
Why abstain from emetic drugs?
“As long as emetogenic drugs are part of the anesthetic regimen, the use of anti-emetics is of limited utility.” —Christian Apfel
Introduction
Facial cosmetic procedures are for vanity purposes compared to medically indicated surgeries like cleft palate or burn repairs. See Table 1 This difference has implications for acceptable versus unacceptable anesthesia risk. Table 1 surgeries can be performed solely under local anesthesia. A characteristic of cosmetic surgeries is the surgeon’s pre-incision injection of various lidocaine/epinephrine solutions to minimize blood loss with vasoconstriction as well as facilitating the dissection. The lidocaine in the lidocaine/epinephrine injections also can provide complete analgesia. Surgeons believe vasoconstriction equals adequate analgesia. With an awake patient, the surgeon may attempt to dismiss patient’s pain complaints as pressure not pain. Patient movement under intravenous (IV) sedation is a more challenging issue to resolve. Is the movement purposeful or non-purposeful? What is the best response to either movement? General anesthesia (GA) often includes the use of muscle blocking agents. Patient movement under GA is rarely an issue. The sedated/anesthetized brain cannot differentiate between the malevolent intent of a mugger’s knife and therapeutic intent of the surgeon’s scalpel. The brain only recognizes bodily invasion (Figure 2).
Table 1
| Brow lift, open or endoscopic |
| Rhytidectomy, open or closed |
| Blepharoplasty |
| Rhinoplasty |
| Otoplasty |
| Facial implants |
| Fat transfer |
| Facial resurfacing, mechanical abrasion, chemical peel or laser |
| Bichat fat pad excision |
| Platysma band plication |
Measure the brain
Many patients prefer to have some consciousness alteration during their cosmetic surgery. The remaining anesthesia choices are IV sedation plus local analgesia or GA plus local analgesia. Møiniche et al. published minimal preemptive analgesia achieved with pre-incision lidocaine/epinephrine injections after GA induction (2).
Why not the convenience of GA? The expense of an anesthesia machine equipped with exhaust gas scavenging must be borne. Isoflurane, sevoflurane, and desflurane as well as succinylcholine are malignant hyperthermia (MH) triggers. Although MH is rare, it has happened (shorturl.at/uINZ3). The facility must be prepared to treat MH with a dantrolene loading dose of 2.5 mg/kg (3). Dantrolene has a three-year shelf life and must be replaced regularly. A newer dantrolene preparation (RyanodexTM) is reported to be much faster to prepare and requires less volume in an emergency MH crisis. For many office facilities, the expense of providing GA may be greater than the case load warrants. The rare risk of MH is unacceptable in patients having surgery without medical indication, i.e., elective cosmetic procedures.
Neither propofol nor ketamine are MH triggers, require dantrolene inventory, an anesthesia machine or scavenging. During IV sedation, any patient movement is often interpreted by the surgeon as the sedation being inadequate or ‘too light’. Deeper anesthesia will not resolve the issue of the perception of inadequate sedation, even under GA (4). Opioid inclusive anesthesia (with IV sedation or GA) fails to reduce postoperative pain (5). Not infrequently, the anesthesiologist’s response to the surgeon’s complaint is ‘inject more local.’ Observing vasoconstriction, the surgeon believes the sedation must be inadequate. Contrary to the surgeon’s contention, the anesthesiologist believes the analgesia is inadequate. The stage is set for a contest of wills that poorly serves the patient. If the surgeon resists the anesthesiologist’s re-injection suggestion, the anesthesiologist’s attempts to mollify the surgeon may result in additional IV agents being administered including opioids, ketamine, or propofol more than required for amnesia and sedation. Inaccurate treatment of non-purposeful movement (especially with opioids) may prolong emergence and increase postoperative nausea and vomiting (PONV) (6). If more than a total 200 mg ketamine or ketamine is given in the last 20 minutes of a case, horizontal nystagmus may result upon emergence. Nystagmus with emergence can simulate motion sickness and trigger PONV. Opioid-induced PONV can lead to a hematoma or wound dehiscence (7).
Can ‘too light’ versus more local argument be resolved in a rational, objectively consistent manner that benefits the patient, the surgeon and anesthesiologist? A decapitated chicken still generates movement. Non-purposeful movement can be generated without cortical input. Processed electroencephalogram (pEEG) monitoring can differentiate between cortically generated, purposeful movement (possible awareness with recall) and spinal cord generated, non-purposeful movement (no awareness or recall potential). A pEEG monitor only measures the hypnotic portion of the anesthesia equation ‘hypnosis + analgesia’ (8).
Analgesia monitors?
Recently, two monitors; namely, analgesia nociception index (ANI, MDolorisTM), and nociception level (NOL, MedasenseTM), have become available purporting to reflect analgesia, or lack thereof, by analyzing the balance between sympathetic and parasympathetic tone in the heart rate (HR). Awareness is a cortical function. Changes in HR and blood pressure (BP) are notoriously unreliable signs of awareness under anesthesia (9). Both ANI and NOL information are derived from peripheral HR signals, not cortical input. Like awareness, nociception is also a cortical function and may not be reflected in HR variation. Neither ANI nor NOL has been validated in Level I RCTs or in large scale clinical trials. It remains to be seen if either, or both, of these devices can deliver on their promise.
pEEG monitors
The first practical pEEG monitor, the bispectral (BISTM) index (Covidien, USA) was approved in 1996 by the US Food and Drug Administration. BISTM values have no units. See Table 2 Similar devices like SedLineTM (Masimo, USA), EntropyTM (GE Healthcare, USA) and others have subsequently become available. No other pEEG maker has a publication demonstrating superiority to the BISTM monitor. What level of consciousness (sedation or hypnosis) is required to provide adequate operating conditions for the surgeon while meeting the patients’ demand for amnesia and safety?
Table 2
| Index value | Level of sedation |
|---|---|
| 98–100 | Awake |
| 60–75* | Moderate to deep sedation |
| 45–60 | Hypnosis compatible with general anesthesia |
| <45 | Over medicated |
*, with baseline [26–30] electromyogram values. BIS, bispectral index.
Pain and PONV outcomes
Pain and PONV remain the two most common causes for unexpected hospitalization after day surgery (10). No midazolam premedication was used in a propofol-only sedation paradigm. See Table 3 Over Friedberg’s 20-year experience with pEEG monitored, opioid-free, propofol then 50 mg ketamine, then subcutaneous local analgesia paradigm, not one of more than 4,000 patients were hospitalized for pain or PONV. No patient experienced hallucinations or awareness with recall (11,12). All patients had commercial insurance coverage. In Switzerland, Fangio et al. reported similar opioid-free success with Friedberg’s paradigm (13). Additionally, no opioid addicts were created. No opioid overdose deaths occurred. The overwhelming majority of patients were discharged to home after one postoperative hour. No professional aftercare givers were required (12).
Table 3
| Pleasant induction; happy drugs for happy surgery |
| Spontaneous ventilation/patent airway maintenance |
| Minimal airway support required |
| Endotracheal intubation avoided |
| Difficult airway avoided |
| Outliers identified |
| Differential diagnosis of purposeful versus non-purposeful movement |
| More cost-effective propofol administration |
Consensus PONV prevention guidelines suggest high-risk patients receive multiple anti-emetics (14). From 1992 to 1998 and prior to pEEG monitoring, 1,264 opioid-free, propofol ketamine patients had propofol incrementally titrated to loss of lid reflex and loss of verbal response prior to ketamine administration (15). The majority of these patients were Apfel-defined, high PONV risk, i.e., nonsmoking, females with previous PONV or motion sickness history, having emetogenic (cosmetic) surgery. Despite having been given no antiemetics, these opioid-free patients had a 0.6% PONV rate (15)!
“If you want patients to stop throwing up, stop giving them (opioid) medications that make them sick to their stomach.” —Chris Pollock, MB.
Propofol induction
After 1998, propofol induction was with incrementally titrated (https://lnkd.in/gAnPtSD) to BISTM between 60 to 75 with baseline EMG (or 26–30 on the vertical, right-hand scale) (12). Patients often express the pleasantness of the induction. Alternatively, the abrupt induction with the more commonly given 1,000–2,000 mcg/kg propofol bolus produces unconsciousness too rapidly for patients to appreciate this unique propofol quality. A pleasant, incremental propofol induction is a compliment to the patients’ desires for improved appearance from cosmetic surgery. Even obstructive sleep apnea (OSA) patients arrive to the operating room breathing spontaneously through patent airways. Incremental propofol induction preserves both spontaneous ventilation and patent airways. Bolus propofol induction creates the difficult airway (16).
Airway management
Between 1992 to 2018, none of more than 6,000 opioid-free patients incrementally induced with propofol required endotracheal intubation. Another value proposition of incremental propofol induction is that more than 50% of patients did not require any artificial airway support. Avoiding airway instrumentation eliminates potential sore throat complaints in complaint-prone cosmetic surgery patients. About 30% of incrementally induced patients required only rhytidectomy positioning to keep the tongue off the back of the throat to support a patent airway, i.e., chin up, head turned laterally. Another 30% had an unheated IV bag placed under their shoulders to increase the force of extension on the genioglossus muscle (Figure 3). Another 30% had a nasal airway inserted to maintain patency. Compared with oral airways, nasal airways stimulate less of a cough reflex. About 10% required laryngeal mask airway (LMA) support. Only for rhinoplasty was a flexible LMA routinely inserted and taped to the chin to optimize the surgeon’s field visualization (Figure 4). Less anesthetic trespass is required with an LMA compared with an endotracheal tube.
In addition to avoiding difficult airway creation, incremental induction facilitates rapid identification of outliers. Most patients were able to achieve moderate to deep sedation (i.e., 60< BISTM <75 with baseline EMG) with a propofol infusion rate between 25–50 mcg/kg/min. Extremely fragile patients only required an infusion rate of 2 mcg/kg/min rate to achieve the same numerically reproducible level of sedation! Alternatively, extremely resistant patients required up to 200 mcg/kg/min for the same numerical level of sedation (12). Without directly measuring cortical propofol response, it would be very challenging to appreciate the hundred-fold variation in sensitivity to propofol. Over medicating the fragile patient leads to prolonged emergence and discharge. While under-medicating the resistant patient leads to a difficult surgical field as well as the addition of more agents that may ultimately also prolong emergence and discharge. Appropriately medicating these challenging patients with BISTM/EMG monitoring is a more science-based, cost-effective practice (12). Incremental propofol induction has multiple value propositions including a statistically significant 30% reduction in propofol administration (17) (Table 3).
Nuts and bolts
A Medfusion 2010i infusion pump was used for propofol titration (Figure 5). The initial base infusion rate was set at 25 mcg/kg/min and the initial bolus was set at 50 mcg/kg. Repeated, sequential 50 mcg/kg propofol boluses were administered until a decrease in real time EMG was observed. The real time EMG trend will decrease before the 15−30 seconds’ delayed BIS value. Most patients achieved a BISTM value of less than 75 but greater than 60 with baseline EMG in less than 3 minutes. (https://lnkd.in/gAnPtSD) With induction, once the EMG trend falls to baseline (or 26–30) but the BIS value continues to decrease below 60, it is necessary to decrease the base rate to a level that maintains the BIS level above 60 but below 75. Conversely, if the BIS level remains above 75 with a baseline EMG, it will be necessary to increase the base infusion rate and consider increasing the base rate and increase the bolus to 75 or 100 mcg/kg to decrease the BIS below 75 but above 60.
Differentiation between non-purposeful and purposeful movement
With patient movement without EMG spikes while propofol is titrated to BISTM between 60 and 75, the anesthesiologist can assure the surgeon this movement is non-purposeful. With these BISTM/EMG values, the patient is unconscious and amnestic. Additional local anesthesia in the immediate area of dissection will terminate 98–99% of patient movement despite the presence of vasoconstriction (12). BISTM/EMG monitoring differentiates non-purposeful from purposeful movement and scientifically demonstrates vasoconstriction does not guarantee adequate analgesia (18). The anesthesiologist’s challenge lies in convincing the surgeon of the value of momentarily stopping the dissection and re-inject a bit more local. Preoperative adjustments of the surgeon’s expectations facilitate this interruption to non-purposeful movement during sedation (19).
Alternatively, when EMG spikes occur without patient movement, the anesthesiologist must recognize incipient arousal (20). The anesthesiologist should react with the same alacrity as with HR or BP changes. Supplemental propofol, typically in 100–200 mcg/kg sequential boluses are given to lower the EMG spike back to baseline level while avoiding giving too much too quickly and producing apnea. Following this additional propofol administration, minimal HR or BP perturbations occur. The BIS value will remain within the desired 60 to 75 range.
The main limitation of the BISTM monitor is the time required to process the signal. BISTM values are delayed 15–30 seconds from real time (Figure 6). Kindly note very well, the EMG (red) trend decreases before the BISTM (yellow) trend. The electromyogram (EMG) of the facial frontalis muscle is as instantaneous as the EKG of the cardiac muscle. Even patients who have forehead BotoxTM generate a sufficient signal to make EMG trending useful. Also, the plug-in BIS modules do not have the software to display EMG trending compared with free-standing BIS units. Although anesthesiologists can follow a numerical increase in the EMG value with plug-in BISTM modules, they more readily process information from the left to right sweep on the vital signs monitor. Friedberg’s experience was exclusively with a free-standing BIS unit. EMG spikes signal incipient arousal (20). Arousal precedes nociception. Preventing arousal prevents secondary nociception (1). Picture a hand touching a hot stove. Withdrawal comes first, then ‘ouch,’ not the other way around.
Challenging cases
How can one perform a rhytidectomy of browlift with a BISTM sensor on the forehead? Unless foreign bodies like cheek implants or brow fixation screws are used, neither procedure is sterile. If the surgeon is concerned, the sensor should only be prepped with a dabbing motion. Vigorous scrubbing will dissolve the adhesive and cause failure of sensor adhesion. Nicanor Isse is one of three American plastic surgeons credited with developing the endoscopic browlift (Figure 7).
In Isse’s Newport Beach and Burbank, California offices, Friedberg used a BISTM monitor on every one of his browlift and rhytidectomy patients without a single surgical site infection (SSI) between 1999 through 2004. For rhytidectomies, the #3 BIS Quatro sensor can alternatively be placed on either the forehead or the temple area to avoid the pre-auricular incision. See Figure 8 for browlift patients, only during the relatively brief interval when the flap is completely elevated, does the loss of contact cause the BISTM transiently fail to provide a value.
Textbook
In 2004, Friedberg began work on his Cambridge University Press textbook, ‘Anesthesia in Cosmetic Surgery’ (Figure 9). This was the first anesthesia textbook in the field, the first to display a pEEG monitor on the cover and the first to numerically define levels of sedation/anesthesia instead of verbally describing them (Table 2). First published in English in 2007, the textbook was later translated into Portuguese in 2009 and into Mandarin in 2015. The US military adopted Friedberg’s mobile propofol ketamine, room air, spontaneous ventilation paradigm to enable anesthesia care in forward units without needing anesthesia machines or large H oxygen tanks. Chapter 7 ‘Propofol ketamine beyond cosmetic surgery, implications for military medicine and mass-casualty anesthesia’ was written by the US military. For his more mobile anesthesia paradigm, Friedberg received a US Congressional special recognition award (Figure 10).
Facility level of care delineated
US office-based cosmetic surgery suites are classified into 3 groups: Level I Local anesthesia only, Level II IV sedation, Level III GA. Being able to define and differentiate between IV sedation and GA is not an inconsequential matter. Anesthesia may be defined as the sum of hypnosis plus analgesia (8). ‘Hypnosis’ includes amnesia/sedation. ‘Analgesia’ includes sufficient relaxation to imbricate rectus muscle sheaths for abdominoplasty or dissect the pectoralis muscle from the chest wall for sub-pectoral breast augmentation. When inhalation anesthesia is administered for GA, generalized hypnosis and generalized analgesia is simultaneously provided. Administering systemic opioids also provides generalized analgesia and transforms an IV sedation or a little monitored anesthesia care (MAC) into IV GA or big MAC. A possible implication of this transformation could be to increase from a Level II to a Level III facility and require this type of facility to incur the expense of having an anesthesia machine, provide scavenging when no inhalational agents are administered, and stock dantrolene. Propofol is a hypnotic agent. Ketamine is a dissociative agent. Neither propofol nor ketamine are analgesic agents. Propofol ketamine sedation is appropriate for Level II facilities.
Ketamine
Following establishment of a stable CNS propofol level, a 50 mg ketamine dose was administered 2–3 minutes prior to multiple local anesthetic injections (11,12). Ketamine is an NMDA receptor blocking agent. The dissociative effect or immobility to noxious stimulation reflects NMDA receptor saturation. Beginning in 1992 through 2018 in more than 6,000 opioid-free patients, 50 mg ketamine dose produced 10–20 minutes’ immobility to multiple local injections in patents weighing between 30 up to 145 kg. The largest patient immobilized with 50 mg ketamine is pictured (See Figure 11). Patients ages 7 up to 94 also received the same 50 mg ketamine dose with the identical immobility outcome (21). The number of NMDA receptors does not appear to vary with body weight or age. Immobility reflects NMDA receptor saturation, not merely blocking an indeterminate number of receptors (21). Failure to block NMDA receptors is why preemptive analgesia fails with opioids nor inhalation anesthetics. Absence of EMG spikes with multiple local anesthetic injections is prima facie evidence of NMDA receptor saturation (dissociation) and the beginning of preemptive analgesia. The 50 mg ketamine dose prevents patients’ brains recognizing the surgeon’s bodily invasion with local anesthesia injection and precludes internal pain fibers going on high alert. Local anesthesia administered after ketamine dissociation prolongs the initial ketamine deception.
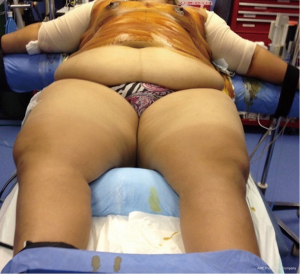
A note of caution is warranted. Ketamine-associated laryngospasm is a very rare but distressing event. It is triggered by secretions contacting the vocal cords. To minimize ketamine-associated secretions, glycopyrrolate 0.2 mg IV was always administered prior to propofol induction. See Table 4 Glycopyrrolate is preferred over atropine. Atropine causes more tachycardia than glycopyrrolate. Lidocaine/epinephrine injections also frequently produce tachycardia. Unexplained tachycardia is often the only sign of a MH event. The typical ‘crowing’ of partially closed cords is absent. The vocal cords are completely closed with ketamine-associated laryngospasm. A cough or sneeze is the only prodrome. Larson’s maneuver, i.e. anterior jaw thrust with positive pressure ventilation, does not satisfactorily resolve this uncommon type of laryngospasm. Lidocaine 1 mg/lb or 2 mg/kg reliably resolves this spasm (22).
Table 4
| Glycopyrrolate 0.2 mg IV |
| Propofol titrated to BIS <75 with baseline EMG |
| Ketamine 50 mg IV |
| Dexamethasone 10 mg IV |
| Local anesthesia by surgeon |
| Rx Laryngospasm… stat IV lidocaine 1 mg/pound with cough or sneeze |
| Browlift: pre-closure… supraorbital ridge infiltration with 0.25 bupivacaine |
IV, intravenous; BIS, bispectral index; EMG, electromyogram.
Local analgesia
What level of analgesia is effective for facial cosmetic surgeries? Tumescent analgesia with 0.05% lidocaine and 1:1M epinephrine, performs well for rhytidectomies with 50–100 ccs injected per side. Other surgeons inject with 0.5% lidocaine and 1:200,000 epinephrine, 50–100 ccs per facial side. In the author’s early experience, some surgeons refrained from injecting the contralateral side until they completed the initial side’s dissection. They believed the vasoconstriction and analgesia would dissipate. Injections of preciously un-injected (or ‘virgin’) fields require an additional 50 mg ketamine dose. Re-injection of previously injected surgical fields does not require additional ketamine to produce immobility. An informal review of 1,000 cases revealed 80% were performed with a single or two 50 mg ketamine doses (15). As experience with the technique grew, surgeons were encouraged to inject both sides of the face with the initial 50 mg ketamine dose. Both vasoconstriction and analgesia remained satisfactory. The need for re-injection was very rare. Even after an average 4- to 5-hour rhytidectomy, neither additional injections nor additional ketamine was needed. For rhinoplasty and blepharoplasty, analgesia with 1% lidocaine with 1:100,000 epinephrine was often inadequate. Smaller volumes of 2% lidocaine with 1:100,000 epinephrine provided more consistent analgesia. Facial resurfacing cases were performed with facial nerve blocks after the ketamine was administered. Pre-emergence, postoperative infiltration of the supraorbital ridge with 0.5% bupivacaine was effective in preventing postoperative headache complaints after browlifts. Compared to body cosmetic surgeries, the relatively small amounts of 2% lidocaine with epinephrine for rhinoplasty and blepharoplasty make local anesthetic toxicity unlikely.
Conclusions
Opioid free, propofol ketamine IV sedation guided by Friedberg’s Triad has advantages over either local only or GA plus local. Patients do not hear, feel, or remember their surgery. For the surgeon, the operative field approximates GA conditions without the greater pharmacologic trespass, need for an anesthesia machine, scavenging, or MH risk The persistent problems of pain and PONV are virtually eliminated. Opioid-free, propofol ketamine sedation meets the specifications of a Level II facility. Ketamine hallucination fears are eliminated with hypnotic levels of propofol. BISTM/EMG monitored cortical response introduces the scientific practice of reproducibility across the hundred-fold variation in propofol requirement at the same numerical sedation level. BISTM/EMG monitoring objectively resolves the dilemma of ‘too light’ versus ‘needs more local’ that prevents more elective cosmetic surgery being performed with IV sedation.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://joma.amegroups.com/article/view/10.21037/joma-22-17/coif). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Friedberg BL. Tríada de Friedberg, Friedberg’s Triad, a pathway to opioid free anesthesia (OFA) and better outcomes. MPJ 2021. doi:
10.20986/mpj.2021.1004/2021 10.20986/mpj.2021.1004/2021 - Møiniche S, Kehlet H, Dahl JB. A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: the role of timing of analgesia. Anesthesiology 2002;96:725-41. [Crossref] [PubMed]
- Malignant Hyperthermia Association of the United States.How Much Dantrolene Should Be Kept On Hand? Available online: https://www.mhaus.org/faqs/how-much-dantrolene-should-be-kept-on-hand/
- Lichtner G, Auksztulewicz R, Velten H, et al. Nociceptive activation in spinal cord and brain persists during deep general anaesthesia. Br J Anaesth 2018;121:291-302. [Crossref] [PubMed]
- Frauenknecht J, Kirkham KR, Jacot-Guillarmod A, et al. Analgesic impact of intra-operative opioids vs. opioid-free anaesthesia: a systematic review and meta-analysis. Anaesthesia 2019;74:651-62. [Crossref] [PubMed]
- Friedberg BL, Friedberg BL. Postoperative pain, nausea and vomiting need not continue to plague our patients. Anaesth Pain Intensive Care 2017;21:399-401.
- Eryilmaz T, Sencan A, Camgoz N, et al. A challenging problem that concerns the aesthetic surgeon: postoperative nausea and vomiting. Ann Plast Surg 2008;61:489-91. [Crossref] [PubMed]
- Friedberg BL. What is general anesthesia? Plast Reconstr Surg. 2010;125:222e-3e. [Crossref] [PubMed]
- Domino KB, Posner KL, Caplan RA, et al. Awareness during anesthesia: a closed claims analysis. Anesthesiology 1999;90:1053-61. [Crossref] [PubMed]
- Aubrun F, Ecoffey C, Benhamou D, et al. Perioperative pain and post-operative nausea and vomiting (PONV) management after day-case surgery: The SFAR-OPERA national study. Anaesth Crit Care Pain Med 2019;38:223-9. [Crossref] [PubMed]
- Friedberg BL. Hypnosis first, then dissociation. Anesth Analg 2003;96:913-4. [PubMed]
- Friedberg BL. BIS Monitoring Transformed Opioid-Free Propofol Ketamine Anesthesia From Art to Science for Ambulatory Cosmetic Surgery. Aesthetic Plast Surg 2020;44:2308-11. [Crossref] [PubMed]
- Fangio P, Bayol JC, Lê-Huu S, et al. Let's Avoid Opioids in Aesthetic Surgery. Plast Reconstr Surg 2020;146:241e-3e. [Crossref] [PubMed]
- Gan TJ, Belani KG, Bergese S, et al. Fourth Consensus Guidelines for the Management of Postoperative Nausea and Vomiting. Anesth Analg 2020;131:411-48. [Crossref] [PubMed]
- Friedberg BL. Propofol-ketamine technique: dissociative anesthesia for office surgery (a 5-year review of 1264 cases). Aesthetic Plast Surg 1999;23:70-5. [Crossref] [PubMed]
- Friedberg BL. The difficult airway in office-based anesthesia. Plast Reconstr Surg 2010;125:221e-2e. [Crossref] [PubMed]
- Friedberg BL, Sigl JC. Clonidine premedication decreases propofol consumption during bispectral index (BIS) monitored propofol-ketamine technique for office-based surgery. Dermatol Surg 2000;26:848-52. [Crossref] [PubMed]
- Friedberg BL. Postoperative Nausea and Vomiting with Plastic Surgery: A Practical Advisory to Etiology, Impact, and Treatment. Plast Reconstr Surg 2018;142:608e-9e. [Crossref] [PubMed]
- Friedberg BL. Preoperative instructions, intra-operative environment chapter. In: Friedberg BL. editor. Anesthesia in Cosmetic Surgery. New York: Cambridge University Press; 2007:15-23.
- American Society of Anesthesiologists Task Force on Intraoperative Awareness. Practice advisory for intraoperative awareness and brain function monitoring: a report by the american society of anesthesiologists task force on intraoperative awareness. Anesthesiology 2006;104:847-64. [Crossref] [PubMed]
- Friedberg BL. Ketamine hallucination & dose limits rebutted. Transl Perioper Pain Med 2020;7:170.
- Friedberg BL. Ketamine associated laryngospasm during processed EEG monitored propofol sedation. Transl Perioper Pain Med 2020;7:291-3.
Cite this article as: Friedberg BL. Opioid free anesthesia for facial cosmetic surgery with Friedberg’s Triad. J Oral Maxillofac Anesth 2022;1:11.












