
Nerve and ganglion blocks in the management of headache disorders: a narrative review
Introduction
Headaches, facial pain, and related entities form a significant source of pain, morbidity, and reduced quality of life for the general population worldwide. The International Headache Society (IHS) came out with its latest classification of headaches in 2018 (1). Since then, there has been an explosive number of manuscripts published on various aspects of headaches. Medications form a large component of medical management of many headaches. Consequently, there are numerous newer medications available in the market over the last few decades. These include many newer triptans, gepants, and other medications that were approved for migraines (2-4). However, various systemic and other factors may preclude the use of medications. Some patients may be refractory to the conventional methods of management (5-9). For some of these refractory cases, various surgical ablative and other invasive procedures have been proposed. In a selected group of these headache cases, considerable methods of peripheral nerve blocks and nerve stimulation can significantly positively affect the diagnosis, prognosis, and quality of life of the patient. In this article, we review the evidence, risks, techniques, and landmarks of nerve and ganglion block procedures, that have been employed for headaches, neuralgias, and other pain entities. The readers are encouraged to refer to the cited articles in this manuscript, for the details of the procedural techniques. The IHS-3 has included trigeminal and other craniofacial neuralgias under its classification of headaches (1). Therefore, although for a practicing clinician, neuralgias may not confirm the traditional definition of ‘headache’, we have included a brief section under the utility of nerve blocks for management of neuralgias as well.
The rationale for using peripheral nerve blocks stem from the ability of local anesthesia to block afferent pain sensations. Various pharmaceutical agents including local anesthetics (LAs) and steroids have been used singularly or in combination for these procedures. Many of the LAs bring about good conduction block of pain sensations without appreciably blocking the motor function. The properties of LA such as the onset of action and duration of anesthesia may affect the level of pain relief. Caution should also be expressed in the possible placebo effect that may come with the anesthetic blockage of pain, and the resultant numbness (9,10).
There seems to be no real consensus on the absolute indication and the timing of the use of peripheral nerve blocks in the management of headaches (11,12). There is also considerable variability in the actual anesthetic drug, its concentration, the amount used, the frequencies of the blocks, the proposed time interval between consecutive blocks, and whether to use anesthetics with corticosteroids (11,13-15). Further, an added benefit from blocking an afferent nerve in the head and neck maybe while virtue of its positive effect on pain emanating from other anatomic regions outside the area of supply of the same nerve (case in point: the phenomena of convergence). The procedure is minimally invasive and may be of great therapeutic and management value in many patients suffering from headaches (11). Peripheral nerve blocks are mostly well tolerated, readily accepted by patients and mostly associated with minimal or no serious side effects (11,13) and adverse effects (11,16-20). Many of the nerve block procedures lack true rigorously controlled studies, and some may have been anecdotally reported.
As alluded to earlier, the craniofacial neuralgias deserve special mention. The inclusion of these entities under headaches by the IHS may have everything to do with the distribution of pain being in the head and neck region. It must be noted that the duration of pain in neuralgias may not be consistent with the common concept of ‘headaches’. However, entities like occipital neuralgia, presumably due to the larger area of the head and neck involved in the pain, and due to the duration and triggers, may possibly be seen as headaches by patients and clinicians. In the case of trigeminal and glossopharyngeal neuralgias, it is common practice for the patient to either be referred or self-referred to the dentist/orofacial pain specialist due to the possible ‘dental’ nature of the pain symptoms. We present the following article in accordance with the Narrative Review reporting checklist (available at https://joma.amegroups.com/article/view/10.21037/joma-22-6/rc).
Methods
The detailed method used to identify potential literatures is summarized in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 1st November 2021 to 20th January 2022 |
| Databases and other sources searched | PubMed, Ovid, Science Direct, and textbooks |
| Search terms used (Table S1) | Nerve blocks, headaches, pain management, migraine, individual pain entities, individual nerve blocks and ganglion blocks |
| Timeframe | January 1st 1992 to January 1st 2022 |
| Inclusion and exclusion criteria | Inclusion criteria |
| (I) Articles describing nerve blocks related to orofacial pain in general, and management of headaches in particular | |
| (II) Articles published in English between 1992 and 2022 | |
| (III) Articles whose complete form/PDFs are available | |
| Exclusion criteria | |
| (I) Articles in language other than English | |
| (II) Articles published prior to 1992 | |
| (III) Articles whose complete form was not available | |
| Selection process | All the authors (DCT, DC, SP, PKP, SDM, BCM) conducted independent searches; the results were discussed as a group; selection decisions were unanimous; any differences of opinion were reconciled in discussion with the first author DCT |
Occipital nerve block
Occipital nerve block has been variably described as a lesser occipital (C3) block or greater occipital (C2) or a combined (C2-C3) block (21-24).
Nerve anatomy and distribution
The sensory nerves arising from the dorsal roots at levels C2 and C3 of the spinal cord comprise the ‘occipital nerves’ (25,26). These nerves innervate the scalp from its posterior part to the vertex, and encompasses other anatomic structures including the external ear (26). C2 is known as greater occipital, C3 is known as lesser occipital, and a branch of C3 that supplies C2-C3 facet joint (27) is known as third occipital nerve (Figure 1A) (25). The greater occipital nerve (GON) C2 originates between C1 and C2 vertebrae, and proceeds between two muscles namely inferior oblique and semispinalis capitis (26). This nerve penetrates the semispinalis capitis and sometimes the trapezius and the inferior oblique and is associated with sternocleidomastoid (21,25,26). The lesser occipital nerve (LON) innervates the scalp in the lateral region of the head behind the ear and the cranial surface of the ear (25).
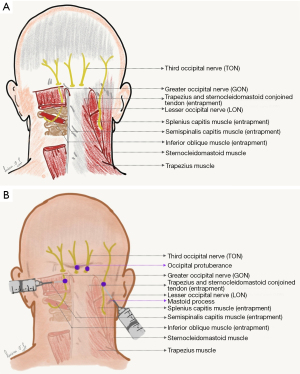
Indications
The indications for these blocks vary widely in the literature. Some of the literature refers to the GON block as routinely used for two conditions, namely occipital neuralgia and cervicogenic headaches. It should be noted that the GON block could be diagnostic, prognostic and/or therapeutic in these conditions (1,9,12,22). GON blocks are also effectively used for the management of cluster headaches and migraines. GON block is also used for diagnostic and therapeutic purposes in cases of C2 entrapment neuropathy (25). Other indications for this GON block include cluster, migraine, chronic daily headache, hemicrania continua, new daily persistent headache, post traumatic headache, post-dural puncture headache, and trigeminal neuralgia (28-36). Occipital nerve block is one of the preferred methods for management of refractory occipital neuralgia (23). The use of GON block in neuralgias of nerve that are anatomically distinct, i.e., trigeminal neuralgia, stems from the explanation that both these entities show convergence at trigeminal nucleus caudalis level (22,25,37,38). Used alone or with steroids, the success rate for pain relief from GON blocks is reported to range from 15 to 35 % in terms of sustained prolonged pain relief extending over several months (16,25,39). The explanation for this benefit given in the literature is the inhibition of the afferent barrage to the nucleus caudalis of the trigeminal nerve and cervical dorsal horn via the GON (37,39). Abortive management of migraine with aura includes bilateral GON blocks (40).
Indications for GON block
Indications for GON block are occipital neuralgia, entrapment neuropathy, cervicogenic headache, cluster (episodic & chronic), migraine (episodic & chronic), status migrainosus, chronic daily headache, hemicrania continua, new daily persistent headache, posttraumatic headache, post-dural puncture headache, trigeminal neuralgia (28-36).
Landmarks
A straight line connecting the occipital protuberance and the mastoid process forms the anatomic landmark of the GON (27).
Technique
Various techniques have been described in the literature for GON blocks. One of the most accepted methods of GON block involves an LA injection at a tender point, that is lateral to the occipital protuberance. The LON block is administered approximately 1.5 inches lateral to the palpated occipital artery (Figure 1B) (14,41-46).
It has been proven that use of ultrasound may help to inject the anesthetic block at a more targeted site between C1/C2, although, the benefit of this in comparison to a block given without any image guidance is yet to be proven (39,47,48).
Pharmacology
Much of the literature focuses on the use of either 1–2% lidocaine or 0.25% to 0.5% bupivacaine (23,46). Botulinum toxin-A (Botox) apparently gives longer pain relief than when the blocks are administered with LA alone (49,50). The proposed mechanisms of action of Botox bringing about sustained pain relief in GON blocks have included inhibition of substance P, CGRP, and glutamate (49,51-53).
Adverse effects
Presumably, the adverse/side effect of a GON block may stem from direct trauma to the local tissues/nerve, pharmacologic side effect of the agent, or injury to an adjacent blood vessel causing localized bleeding. It must be noted that most of these adverse effects are of a transient and mild nature. The rarely reported side effects include hematoma, pain at the site of injection, alopecia, and local injection site infection (54). The clinician should be aware of the possibility of more pronounced localized side effects with repeated injections, especially with steroids. Very rarely steroids can cause local tissue necrosis, wasting of muscle and a risk of rupture of the tendon (24,55). Bilateral blocks have shown to have higher incidence of side effects. There is a possibility of scalp tissue necrosis secondary to significant vasoconstriction associated with anesthetic solutions containing vasoconstrictors. This effect has been proposed to be the result of vasoconstriction of the occipital artery (28).
Third occipital nerve
The third occipital nerve (TON) originates from the dorsal ramus of the C3 nerve (25). The TON has an anatomic relationship with the C2-C3 facet joint. The TON is known to pierce the splenius capitis, trapezius, and semispinalis capitis muscles. This nerve also supplies sensory innervation to the C2/C3 facet joint (26). Some literature proposes the use of a TON block for accelerated decelerated injury (whiplash) associated pain (9,56,57). It is worth mentioning that C3 stimulation can also be used for management of occipital neuralgia (22,58-61). One of the more common uses of the TON block is for upper cervical pain and cervicogenic headache (62).
Sphenopalatine ganglion (SPG) block
Nerve anatomy and distribution
The SPG is located in the pterygopalatine fossa (PPF). It has autonomic, sensory and motor components (63). The Vidian nerve carries the sympathetic and parasympathetic nerve fibers to the SPG. Synapse of preganglionic parasympathetic neurons occurs within the SPG, and postganglionic parasympathetic fibers then reach the target organs (lacrimal gland, nasal glands, palatine glands, and pharyngeal glands) via the SPG, through the ophthalmic and maxillary divisions of the fifth nerve. Preganglionic sympathetic nerve fibers first synapse in the superior cervical ganglion, and the postganglionic sympathetic nerve fibers travel along with parasympathetic fibers and pass through the SPG (with no synapsing) (63). The sensory component of SPG is depicted in Table 2.
Table 2
| Nerve branches | Anatomic distribution (sensory) |
|---|---|
| Orbital | Periosteum of orbit |
| Greater palatine/lesser palatine | hard and soft palates; palatine tonsil (mucosa) |
| Posterior lateral nasal; nasopalatine | Mucosa of: |
| Posterior and inferior nasal cavity | |
| Posterior ethmoidal air cells | |
| Hard palate immediately posterior to maxillary incisors | |
| Pharyngeal | Mucosa of nasopharynx, sphenoid sinus, and posterior ethmoidal air cells |
Indications
Cluster headaches are proposed to have activation of the parasympathetic outflow from the superior salivary nucleus of the seventh nerve, mainly via the SPG (65). SPG blocks are effectively used for the management of cluster headaches and migraines (9,66-69). SPG blocks are also effectively used in post-dural puncture headache, post-traumatic headache, and post-herpetic neuralgia (68,70,71). SPG blocks used repetitively are indicated in cases of hemicrania continua in patients intolerant to indomethacin (68).
Landmarks
The PPF can be accessed via the sphenopalatine foramen, which is approachable at the level of nasal mucosa and behind the middle nasal concha (64). The other local landmarks related to the SPG are the middle nasal turbinate and the PPF (pyramidal space formed at the junction of maxilla, palatine and sphenoid bones) (72).
Technique
Initially described by Barré in 1982, the SPG block utilized varying concentrations of cocaine solutions in a trans nasal approach (73). The technique simply involves patients in a supine position, tilting their head upwards, and deposition of the drug in each nostril (74). A similar approach utilizing 4% xylocaine was employed by Kittrelle et al. in 1985 (75), and later reconfirmed by Berger et al. (76). Three traditional approaches are employed in the SPG block. These include the trans-nasal approach, transoral approach and lateral infra-zygomatic approach (77). A newer technique employing the supra-zygomatic approach is also used (78). The transoral approach is a direct approach for blocking palatine nerves by targeting the greater palatine foramen. The PPF can be approached by passing a 27-gauge curved dental needle through the greater palatine foramen medial to the maxillary wisdom tooth to reach the main trunk of V2 division of trigeminal nerve at the superior portion of the PPF (27,77,79). Topical application of LA solution on a cotton tipped applicator to the posterior wall of nasopharynx in the area of middle turbinate with the patient in supine/reclined position is the trans nasal approach for blocking the SPG (Figure 2) (66,73,79). The lateral infra-zygomatic approach to block the SPG is usually guided by fluoroscopy or computed tomography (27,79,80).
Pharmacology
Most commonly used drugs for SPG block include 2% to 4% lidocaine, 0.5% bupivacaine, 4% cocaine, depot steroids or 6% phenol (79).
Adverse effects
The most common adverse effects of SPG block include epistaxis usually with trans-nasal and infra-zygomatic approach (81). Other adverse effects include hematoma due to accidental intravascular injection of maxillary artery or its branches which are in PPF, infection if there is a breach in oral or nasal mucosa during giving the SPG block (27). Transient hypoesthesia of the maxilla, palate or pharynx can occur as sensory supply to these areas are via the SPG block (82,83). Transient diplopia is common after SPG block due to spread of LA solution affecting abducent nerve in the inferior orbital fissure from the pterygopalatine fissure (84).
Stellate ganglion (SG) block
Nerve anatomy and distribution
The SG is formed by the fusion of inferior cervical ganglion and first thoracic sympathetic ganglion (85-89). The traditional description of the location of SG is the junction of C7 vertebrae and T1. The sympathetic supply to head, neck and upper extremity is via the sympathetic nerves that traverse through the SG (90,91). By blocking SG, sympathetic supply to the ipsilateral side of the upper quadrant of the body is inhibited. Location of SG is in front of the transverse process of C7 vertebra and behind the vertebral artery (Figure 3A) (64). Pre-ganglionic sympathetic nerve fibers traverse from spinal cord T2 or T3–T5 to SG. Sweat glands, blood vessels of the upper extremities, nerve branches to lung and heart, and nerve plexus surrounding vertebral artery receive postganglionic sympathetic nerve fibers from SG through spinal nerves C7, C8 and T1 (64,92). The blood supply to head, neck and upper limbs on the ipsilateral side is increased by blocking the SG (93-95). SG block reduces the response to nociception and also has sedative effects, thus resulting in antinociception (96,97).
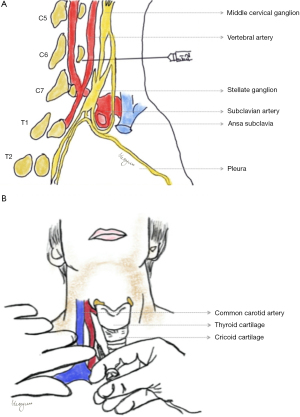
Indications
The SG block has been used to manage complex regional pain syndrome (CRPS I & II) (90,98-100). Some articles mention the use of SG block for CRPS of the craniofacial region as well (101). It is also used for sympathetically maintained pain (98,102,103). It is effectively used to manage painful conditions like herpes zoster and post herpetic neuralgia (89,99,104-107). SG block is also useful in treating cases of refractory migraine and tension type headache (99,108). SG block is also effective in management of intractable atypical facial pain (90,93,109,110) and temporal arteritis (111,112). Recently, it has been used for therapeutic management of burning mouth syndrome (64,113).
Landmarks
The most common landmarks used while giving a SG nerve block are C6 vertebra, Chassaignac’s tubercle on the transverse process of C6 vertebra, the cricoid cartilage and the carotid artery (114). For ultrasound guided technique the soft tissue landmarks used are carotid artery, internal jugular vein, thyroid gland, longus colli, longus capitis muscle, prevertebral fascia, and the root of C6 spinal nerve (114,115). The anatomic landmarks are summarized in Table 3.
Table 3
| Relation | Structures |
|---|---|
| Anterior | Vertebral artery and subclavian artery, common carotid artery, internal jugular vein and vagus nerve |
| Posterior | Transverse process of C7 |
| Anterolateral | Phrenic nerve |
| Anteromedial | Trachea, thyroid, esophagus, recurrent laryngeal nerve |
| Anteroinferior | Apex of lung |
Technique
There are three known methods of SG block. The conventional blind approach of administering SG nerve block is by identification and palpation of the Chassaignac’s tubercle on the transverse process of C6, thereby preventing inadvertent intravascular injection. The injected solution spreads to the SG through the longus colli muscle (90,117). The level at which SG block is given to avoid inadvertent intravascular injection in the vertebral artery or lungs causing pneumothorax is C6 level (Figure 3B) (118,119). Various new techniques have been used to administer SG block such as ultrasound and fluoroscopic guided blocks (119-121). These techniques have made the conventional blind approach almost obsolete. The fluoroscopic guided SG block utilizes only bony landmarks like the transverse process of C6 vertebra. The anterior-posterior approach is the most commonly used approach in fluoroscopic guided technique, wherein the needle is advanced till the bone (tubercle of transverse process of C6) is reached and taken out a few millimeters before injecting (119,122). The ultrasonography (US) guided technique utilizes soft tissue landmarks in addition to bony landmarks for administration of SG block. Unlike fluoroscopic technique, bone is not contacted in ultrasound guided block. In US guided technique, the needle is placed in the fascial plane between the prevertebral fascia which covers the posterior fascial layer of the carotid sheath and the longus colli muscle (119,122).
Pharmacology
The various LA solutions used for administering SG nerve block are 5 mL of 0.5% bupivacaine (123), 0.5% mepivacaine in 5 mL (124), and 5 mL of 1.5% lidocaine (99).
Adverse effects
The most common adverse effects of SG nerve block include intravascular injection in the vertebral artery, carotid artery, internal jugular vein, and inferior thyroid artery. This is more common with the conventional blind technique and fluoroscopic guided SG block (125). Pneumothorax, esophageal and tracheal puncture can occur with fluoroscopic technique (125). The direct visualization of vascular and soft tissue structures such as vertebral, inferior thyroidal, cervical, and carotid arteries, thyroid gland, esophagus, and nerve roots with ultrasound guided SG block makes this procedure relatively safe and minimizes complications seen with other techniques (122,126). Other adverse effects include transient Horner’s syndrome (127,128), hematoma, airway obstruction (129), injury to recurrent laryngeal nerve, vagus nerve and brachial plexus roots (114). Severe hypertension, transient cough, dyspnea, persistent ptosis, hemi diaphragmatic paralysis/phrenic nerve injury, dural puncture, pneumothorax, hematoma and infection (125,128-131).
Supraorbital and supratrochlear nerve blocks (STNs)
Nerve anatomy and distribution
Supraorbital and supratrochlear nerves are the branches of the ophthalmic division (V1) of the trigeminal nerve supplying the orbit and upper eyelids, amongst other structures (9,132-136). They pass through the orbit above the supraorbital ridge providing easy access to neural blockade (9). The supraorbital nerve exits the supraorbital margin through a notch (135,136). The supratrochlear nerve travels along the medial roof of the orbit, between the trochlear and supraorbital foramina exiting through the frontal notch supplying the deep tissues of the forehead (134).
Indications
Supraorbital nerve blocks (SONs) and STNs are indicated in the management of chronic headaches (137). These blocks are also used to effectively manage refractory frontal headaches (138) Other indications for these blocks are the abortive therapy of acute migraine and for management of status migrainosus (139,140). STN block is also used for various autoimmune and inflammatory conditions that involve the trochlear nerve such as cranial neuritis, multiple sclerosis and Tolosa-Hunt syndrome (141). SON block is used to manage swimmers headaches (141). SON and STN blocks are indicated in management of cluster headaches (11). Other indications for SON and STN blocks that appear in the literature are supraorbital and supratrochlear entrapment neuropathies; the diagnosis and management of facial pain; palliative therapy for malignancy; and for pain from herpes zoster and facial bone fractures (27).
Landmarks
The landmarks for SON and STN blocks are supraorbital notch, bony orbit, and the corrugator muscle (11,142-144).
Technique
SON
After identification of supraorbital notch, a 25-gauge needle is inserted at the target area and is advanced towards the midline approximately 15 degrees away from the supraorbital foramen till it touches the periosteum (Figure 4) (9,142,144-147).
STN
Once the medial canthus of the eye is identified, an imaginary line is drawn superiorly from the eyebrow to the medial canthus of the eye inferiorly beneath the eyebrow. A 25-gauge needle is inserted midway on this line and advanced till it reaches the orbital bone and 3cc of anesthetic is deposited after aspiration (Figure 4) (9,144,147,148).
Pharmacology
The most commonly used anesthetic for SON and STN are 0.5% or 1% lidocaine and 0.5% bupivacaine (149-152).
Adverse effects
The most common adverse effects/complications after SON and STN block are hematoma and ecchymosis post injection due to close proximity to vascular structures (141,149).
Facet joint injections
Nerve anatomy and distribution
Facet joint injections are an integral part of medical management of headaches. Facet joint is a synovial joint (cervico zygapophyseal) with a fibrous capsule. The main components of the joint are articular processes of the cervical vertebrae and inferior articular processes of the adjacent vertebrae (153,154). The medial branches of the C4-C8 dorsal rami supply the facet joints (155). A branch of C3 dorsal rami that lies in close proximity of the vertebra, known as the TON supplies the C3-C4 zygapophyseal joint (156). Anesthetic blockade of the TON is utilized in alleviating the pain arising from the C2-C3 joint (27).
Indications
Pain arising from facet joints, neck pain, acceleration-deceleration injury, post-surgical neck pain, pain from cervical degenerative joint disease, cervicogenic headache, other cervical disorders (27,157-160). Some physicians consider the cervical medial branch block as the standard for the diagnosis of facet joint pain (161,162).
Landmarks and technique
Most of the literature consistently talks about the need for fluoroscopy due to the sensitive nature of the structures adjacent to the area of injection. The most commonly used technique for administering facet joint injections is a lateral approach. The patient is made to lie down laterally with a head rest so that lateral flexion of neck is avoided. After identification of facet joint, a 22-to-25-gauge spinal needle is introduced posteriorly, taking care not to advance the needle in the intervertebral foramina and spinal canal. The injectate consists of a combination of LA and steroids. A total of 1 to 1.5 mL of this combination is injected at the target area (163). This procedure is performed under fluoroscopic guidance (157,158,163,164).
Pharmacology
The agents commonly used while administering facet joint injection are a combination of LA and steroids. Either 1% lidocaine or 0.5% bupivacaine is used as an LA. Steroids such as 40% triamcinolone, methylprednisolone, dexamethasone and betamethasone are also used (157,158,165).
Adverse effects
The adverse effects of administering facet joint injections are bleeding, hematoma, intravascular injection in the vertebral artery, dural puncture, meningitis, epidural abscess, vasovagal syncope, pneumothorax, damage to phrenic nerve resulting in palsy, infections such as septic arthritis and psoas abscess (157-159,164,166-168). Other side effects which are transient are swelling and pain at the site of injection (158).
General complications as well as complications pertaining to specific blocks have been summarized in Table 4.
Table 4
| General complications | Drug related complications | Block specific complications | ||
|---|---|---|---|---|
| Blocks | Local complications | Systemic complications | ||
| (I) Bleeding, haematoma, hemorrhage, swelling, dyspnea, paresthesia, hyperesthesia, dysesthesia, mechanical trauma to lip due to paresthesia, nerve injury, facial paralysis, ocular complications like paralysis of extraocular muscles, diplopia, ptosis, miosis, enophthalmos, permanent loss of vision, Horner like syndrome (II) Allergic reactions, methemoglobinemia, seizures, prodromal symptoms like tinnitus, auditory disturbances, confusion, dysphoria, dysarthria, circumoral numbness, metallic taste in mouth, agitation, loss of consciousness. Cardiovascular symptoms like bradycardia, tachycardia, hypotension, hypertension, asystole, ventricular fibrillations (169-174) |
(I) Local—depends on multiple variables including, but not limited to, mode of administration, volume used, patient factors, and specific formulation of the drug. Most common are neurotoxicity, myotoxicity (articaine and bupivacaine), tissue irritation (redness, edema, urticaria), burning, color changes (steroids and botulinum toxin) (II) Systemic—lidocaine has a biphasic action, being an anticonvulsant at lower concentrations, and causing tonic-clonic seizures at higher concentrations. Hypersensitivity reactions with botulinum toxin |
Occipital | Perioral numbness, tinnitus, agitation, metallic taste, coma (175) | Central nervous system: SeizureCardiovascular system: hyper- or hypotension, tachy or bradycardia, ventricular arrhythmia, cardiac arrest, myotoxicity, atrophy, alopecia, hyperpigmentation, folliculitis (175) |
| Sphenopalatine ganglion | Hematoma, epistaxis, intravascular injection, temporary hypoesthesia or dysesthesia in the palate, maxilla, or posterior pharynx (27) | Post-dural puncture headache, permanent paraparesis, cauda equina syndrome, meningitis, and epidural infection (176) Reflex bradycardia, infection, dryness of the eye, temporary diplopia (27) | ||
| Stellate | Hematoma, bleeding, swelling, intravascular injection in vertebral artery, carotid artery and internal jugular vein, dural puncture, esophageal and tracheal puncture (125,128-131) | Severe hypertension, allergic reaction, transient cough, hoarseness, dysphagia, dyspnea and respiratory depression, visual hallucinations, ptosis, bloodshot conjunctiva, seizures, light-headedness, migraine headaches, transient global amnesia, reading difficulty, brachial plexus block, subdural block/intraspinal blockade, transient locked-in syndrome, bilateral sympathetic blockade, decreased contralateral blood flow, contralateral and bilateral Horner’s syndrome, myoclonus arm numbness, lower limb edema, internal jugular vein thrombosis, hemi diaphragmatic paralysis (125) | ||
| Supraorbital & Supratrochlear | Bleeding, intravascular injection, hematoma (177) | Light-headedness, vasovagal syncope, allergy to local anesthetic or corticosteroid, teratogenicity, alopecia, dermal atrophy (177) | ||
| Facet joint injection | Swelling and pain at the site of injection (158) | Paresthesia, numbness, paralysis, vertebral artery damage during cervical entry, neuritic pain, paraspinal abscess, leakage of anesthetic into the spinal canal causing motor and sensory blockade, phrenic palsy from overflow of LA during injection at C3-C6 levels, chemical meningitis, epidural abscess, pneumothorax, transient ataxia and unsteadiness due to partial blockade of upper cervical proprioceptive afferents and the righting reflex from the TON block during cervical injections. Significant vascular and neurological injuries (extremely rare in image-guided injections) (81,158) | ||
LA, local anesthetic; TON, third occipital nerve.
Conclusion and clinical pearls
Nerve blocks appear in the literature as an accepted technique aiding in the diagnosis, prognosis determination, pain relief and management of headache disorders. The nerve blocks provide considerable improvement in the quality of life of patients affected by headaches. These blocks can greatly enhance accurate diagnosis, thereby potentially preventing difficulties in diagnosis and enabling the clinician in succinct pain management. There is a need for further retrospective and prospective studies exploring the efficacies of various types of nerve blocks as compared to other conventional modalities in the management of headache disorders.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Mythili Kalladka) for the series “Orofacial Pain: Diagnostic and Therapeutic Topicals, Nerve Blocks and Trigger Point Injection” published in Journal of Oral and Maxillofacial Anesthesia. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://joma.amegroups.com/article/view/10.21037/joma-22-6/rc
Peer Review File: Available at https://joma.amegroups.com/article/view/10.21037/joma-22-6/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://joma.amegroups.com/article/view/10.21037/joma-22-6/coif). The series “Orofacial Pain: Diagnostic and Therapeutic Topicals, Nerve Blocks and Trigger Point Injection” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38:1-211.
- Maitra A, Mukhopadhyay S, Das A, et al. Newer Horizon for Treatment of Acute Attack of Migraine: Lasmiditan and Ubrogepant. Neurol India 2021;69:1759-62. [Crossref] [PubMed]
- Yang CP, Liang CS, Chang CM, et al. Comparison of New Pharmacologic Agents With Triptans for Treatment of Migraine: A Systematic Review and Meta-analysis. JAMA Netw Open 2021;4:e2128544. [Crossref] [PubMed]
- Berman G, Croop R, Kudrow D, et al. Safety of Rimegepant, an Oral CGRP Receptor Antagonist, Plus CGRP Monoclonal Antibodies for Migraine. Headache 2020;60:1734-42. [Crossref] [PubMed]
- Ornello R, Palmisani S, Murphy M, et al. Sphenopalatine Ganglion Pulsed Radiofrequency for the Treatment of Refractory Chronic SUNCT and SUNA: A Prospective Case Series. Headache 2020;60:938-45. [Crossref] [PubMed]
- Zagami AS. Treatment of the Patient with Refractory Headache. Curr Pain Headache Rep 2018;22:23. [Crossref] [PubMed]
- Lai TH, Wang SJ. Update of Inpatient Treatment for Refractory Chronic Daily Headache. Curr Pain Headache Rep 2016;20:5. [Crossref] [PubMed]
- Wöber C, Wessely PAustrian Consensus Group on Refractory Chronic Migraine. Comment on: Martelletti et al. Refractory chronic migraine: a consensus statement on clinical definition from the European Headache Federation. J Headache Pain 2014;15:77. [Crossref] [PubMed]
- Levin M. Nerve blocks in the treatment of headache. Neurotherapeutics 2010;7:197-203. [Crossref] [PubMed]
- de Craen AJ, Tijssen JG, de Gans J, et al. Placebo effect in the acute treatment of migraine: subcutaneous placebos are better than oral placebos. J Neurol 2000;247:183-8. [Crossref] [PubMed]
- Dach F, Éckeli ÁL, Ferreira Kdos S, et al. Nerve block for the treatment of headaches and cranial neuralgias - a practical approach. Headache 2015;55:59-71. [Crossref] [PubMed]
- Blumenfeld A, Ashkenazi A, Napchan U, et al. Expert consensus recommendations for the performance of peripheral nerve blocks for headaches--a narrative review. Headache 2013;53:437-46. [Crossref] [PubMed]
- Afridi SK, Shields KG, Bhola R, et al. Greater occipital nerve injection in primary headache syndromes--prolonged effects from a single injection. Pain 2006;122:126-9. [Crossref] [PubMed]
- Ashkenazi A, Matro R, Shaw JW, et al. Greater occipital nerve block using local anaesthetics alone or with triamcinolone for transformed migraine: a randomised comparative study. J Neurol Neurosurg Psychiatry 2008;79:415-7. [Crossref] [PubMed]
- Naja ZM, El-Rajab M, Al-Tannir MA, et al. Repetitive occipital nerve blockade for cervicogenic headache: expanded case report of 47 adults. Pain Pract 2006;6:278-84. [Crossref] [PubMed]
- Naja ZM, El-Rajab M, Al-Tannir MA, et al. Occipital nerve blockade for cervicogenic headache: a double-blind randomized controlled clinical trial. Pain Pract 2006;6:89-95. [Crossref] [PubMed]
- Leinisch-Dahlke E, Jürgens T, Bogdahn U, et al. Greater occipital nerve block is ineffective in chronic tension type headache. Cephalalgia 2005;25:704-8. [Crossref] [PubMed]
- Sahai-Srivastava S, Subhani D. Adverse effect profile of lidocaine injections for occipital nerve block in occipital neuralgia. J Headache Pain 2010;11:519-23. [Crossref] [PubMed]
- Han KR, Kim C, Chae YJ, et al. Efficacy and safety of high concentration lidocaine for trigeminal nerve block in patients with trigeminal neuralgia. Int J Clin Pract 2008;62:248-54. [Crossref] [PubMed]
- Ferreira Kdos S, Dach F, Speciali JG. Scar neuromas as triggers for headache after craniotomy: clinical evidence. Arq Neuropsiquiatr 2012;70:206-9. [Crossref] [PubMed]
- Yu M, Wang SM. Anatomy, Head and Neck, Occipital Nerves. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Thomas DC, Patil AG, Sood R, et al. Occipital Neuralgia and Its Management: An Overview. Neurol India 2021;69:S213-8. [Crossref] [PubMed]
- Wamsley CE, Chung M, Amirlak B. Occipital Neuralgia: Advances in the Operative Management. Neurol India 2021;69:S219-27. [Crossref] [PubMed]
- Chowdhury D, Datta D, Mundra A. Role of Greater Occipital Nerve Block in Headache Disorders: A Narrative Review. Neurol India 2021;69:S228-56. [Crossref] [PubMed]
- Choi I, Jeon SR. Neuralgias of the Head: Occipital Neuralgia. J Korean Med Sci 2016;31:479-88. [Crossref] [PubMed]
- Kemp WJ 3rd, Tubbs RS, Cohen-Gadol AA. The innervation of the scalp: A comprehensive review including anatomy, pathology, and neurosurgical correlates. Surg Neurol Int 2011;2:178. [Crossref] [PubMed]
- Narouze SN. Interventional Management of Head and Face Pain Nerve Blocks and Beyond. New York, NY: Springer; 2014.
- Ashkenazi A, Blumenfeld A, Napchan U, et al. Peripheral nerve blocks and trigger point injections in headache management - a systematic review and suggestions for future research. Headache 2010;50:943-52. [Crossref] [PubMed]
- Ashkenazi A, Young WB. The effects of greater occipital nerve block and trigger point injection on brush allodynia and pain in migraine. Headache 2005;45:350-4. [Crossref] [PubMed]
- Bovim G, Sand T. Cervicogenic headache, migraine without aura and tension-type headache. Diagnostic blockade of greater occipital and supra-orbital nerves. Pain 1992;51:43-8. [Crossref] [PubMed]
- Gawel MJ, Rothbart PJ. Occipital nerve block in the management of headache and cervical pain. Cephalalgia 1992;12:9-13. [Crossref] [PubMed]
- Caputi CA, Firetto V. Therapeutic blockade of greater occipital and supraorbital nerves in migraine patients. Headache 1997;37:174-9. [Crossref] [PubMed]
- Ambrosini A, Vandenheede M, Rossi P, et al. Suboccipital injection with a mixture of rapid- and long-acting steroids in cluster headache: a double-blind placebo-controlled study. Pain 2005;118:92-6. [Crossref] [PubMed]
- Peres MF, Stiles MA, Siow HC, et al. Greater occipital nerve blockade for cluster headache. Cephalalgia 2002;22:520-2. [Crossref] [PubMed]
- Bigo A, Delrieu F, Bousser MG. Treatment of vascular pain of the face by methylprednisolone injection into the area of the greater occipital nerve: 16 cases. Rev Neurol (Paris) 1989;145:160-2. [PubMed]
- Busch V, Jakob W, Juergens T, et al. Occipital nerve blockade in chronic cluster headache patients and functional connectivity between trigeminal and occipital nerves. Cephalalgia 2007;27:1206-14. [Crossref] [PubMed]
- Goadsby PJ, Knight YE, Hoskin KL. Stimulation of the greater occipital nerve increases metabolic activity in the trigeminal nucleus caudalis and cervical dorsal horn of the cat. Pain 1997;73:23-8. [Crossref] [PubMed]
- Blake P, Burstein R. Emerging evidence of occipital nerve compression in unremitting head and neck pain. J Headache Pain 2019;20:76. [Crossref] [PubMed]
- Barmherzig R, Kingston W. Occipital Neuralgia and Cervicogenic Headache: Diagnosis and Management. Curr Neurol Neurosci Rep 2019;19:20. [Crossref] [PubMed]
- Shah DR, Dilwali S, Friedman DI. Migraine Aura Without Headache corrected. Curr Pain Headache Rep 2018;22:77. [Crossref] [PubMed]
- Kashipazha D, Nakhostin-Mortazavi A, Mohammadianinejad SE, et al. Preventive effect of greater occipital nerve block on severity and frequency of migraine headache. Glob J Health Sci 2014;6:209-13. [Crossref] [PubMed]
- Dilli E, Halker R, Vargas B, et al. Occipital nerve block for the short-term preventive treatment of migraine: A randomized, double-blinded, placebo-controlled study. Cephalalgia 2015;35:959-68. [Crossref] [PubMed]
- Palamar D, Uluduz D, Saip S, et al. Ultrasound-guided greater occipital nerve block: an efficient technique in chronic refractory migraine without aura? Pain Physician 2015;18:153-62. [Crossref] [PubMed]
- Okmen K, Dagistan Y, Dagistan E, et al. Efficacy of the greater occipital nerve block in recurrent migraine type headaches. Neurol Neurochir Pol 2016;50:151-4. [Crossref] [PubMed]
- Ruiz Piñero M, Mulero Carrillo P, Pedraza Hueso MI, et al. Pericranial nerve blockade as a preventive treatment for migraine: Experience in 60 patients. Neurologia 2016;31:445-51. [PubMed]
- Inan LE, Inan N, Unal-Artık HA, et al. Greater occipital nerve block in migraine prophylaxis: Narrative review. Cephalalgia 2019;39:908-20. [Crossref] [PubMed]
- Narouze S. Occipital Neuralgia Diagnosis and Treatment: The Role of Ultrasound. Headache 2016;56:801-7. [Crossref] [PubMed]
- O'Neill F, Nurmikko T, Sommer C. Other facial neuralgias. Cephalalgia 2017;37:658-69. [Crossref] [PubMed]
- Kapural L, Stillman M, Kapural M, et al. Botulinum toxin occipital nerve block for the treatment of severe occipital neuralgia: a case series. Pain Pract 2007;7:337-40. [Crossref] [PubMed]
- Volcy M, Tepper SJ, Rapoport AM, et al. Botulinum toxin A for the treatment of greater occipital neuralgia and trigeminal neuralgia: a case report with pathophysiological considerations. Cephalalgia 2006;26:336-40. [Crossref] [PubMed]
- Welch MJ, Purkiss JR, Foster KA. Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxins. Toxicon 2000;38:245-58. [Crossref] [PubMed]
- Durham PL, Cady R, Cady R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: implications for migraine therapy. Headache 2004;44:35-42; discussion 42-3. [Crossref] [PubMed]
- Cui M, Khanijou S, Rubino J, et al. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain 2004;107:125-33. [Crossref] [PubMed]
- Blumenfeld A, Ashkenazi A, Evans RW. Occipital and trigeminal nerve blocks for migraine. Headache 2015;55:682-9. [Crossref] [PubMed]
- Voigt CL, Murphy MO. Occipital nerve blocks in the treatment of headaches: safety and efficacy. J Emerg Med 2015;48:115-29. [Crossref] [PubMed]
- Bogduk N, Marsland A. On the concept of third occipital headache. J Neurol Neurosurg Psychiatry 1986;49:775-80. [Crossref] [PubMed]
- Lord SM, Barnsley L, Wallis BJ, et al. Third occipital nerve headache: a prevalence study. J Neurol Neurosurg Psychiatry 1994;57:1187-90. [Crossref] [PubMed]
- Weiner RL, Reed KL. Peripheral neurostimulation for control of intractable occipital neuralgia. Neuromodulation 1999;2:217-21. [Crossref] [PubMed]
- Slavin KV, Nersesyan H, Wess C. Peripheral neurostimulation for treatment of intractable occipital neuralgia. Neurosurgery 2006;58:112-9; discussion 112-9. [Crossref] [PubMed]
- Shaladi A, Crestani F, Saltari R, et al. Percutaneous electrical nerve stimulation of peripheral nerve for the intractable occipital neuralgia. Recenti Prog Med 2008;99:295-301. [PubMed]
- Abhinav K, Park ND, Prakash SK, et al. Novel use of narrow paddle electrodes for occipital nerve stimulation--technical note. Neuromodulation 2013;16:607-9. [Crossref] [PubMed]
- Finlayson RJ, Etheridge JP, Vieira L, et al. A randomized comparison between ultrasound- and fluoroscopy-guided third occipital nerve block. Reg Anesth Pain Med 2013;38:212-7. [Crossref] [PubMed]
- Tepper SJ, Caparso A. Sphenopalatine Ganglion (SPG): Stimulation Mechanism, Safety, and Efficacy. Headache 2017;57:14-28. [Crossref] [PubMed]
- Klein RN, Burk DT, Chase PF. Anatomically and physiologically based guidelines for use of the sphenopalatine ganglion block versus the stellate ganglion block to reduce atypical facial pain. Cranio 2001;19:48-55. [Crossref] [PubMed]
- Goadsby PJ. Pathophysiology of cluster headache: a trigeminal autonomic cephalgia. Lancet Neurol 2002;1:251-7. [Crossref] [PubMed]
- Mojica J, Mo B, Ng A. Sphenopalatine Ganglion Block in the Management of Chronic Headaches. Curr Pain Headache Rep 2017;21:27. [Crossref] [PubMed]
- Tepper SJ, Stillman MJ. Cluster headache: potential options for medically refractory patients (when all else fails). Headache 2013;53:1183-90. [Crossref] [PubMed]
- Androulakis XM, Krebs KA, Ashkenazi A. Hemicrania continua may respond to repetitive sphenopalatine ganglion block: A case report. Headache 2016;56:573-9. [Crossref] [PubMed]
- Maizels M, Scott B, Cohen W, et al. Intranasal lidocaine for treatment of migraine: a randomized, double-blind, controlled trial. JAMA 1996;276:319-21. [Crossref] [PubMed]
- Cohen S, Ramos D, Grubb W, et al. Sphenopalatine ganglion block: a safer alternative to epidural blood patch for postdural puncture headache. Reg Anesth Pain Med 2014;39:563. [Crossref] [PubMed]
- Saberski L, Ahmad M, Wiske P. Sphenopalatine ganglion block for treatment of sinus arrest in postherpetic neuralgia. Headache 1999;39:42-4. [Crossref] [PubMed]
- Tashi S, Purohit BS, Becker M, et al. The pterygopalatine fossa: imaging anatomy, communications, and pathology revisited. Insights Imaging 2016;7:589-99. [Crossref] [PubMed]
- Barré F. Cocaine as an abortive agent in cluster headache. Headache 1982;22:69-73. [Crossref] [PubMed]
- Robbins MS, Robertson CE, Kaplan E, et al. The Sphenopalatine Ganglion: Anatomy, Pathophysiology, and Therapeutic Targeting in Headache. Headache 2016;56:240-58. [Crossref] [PubMed]
- Kittrelle JP, Grouse DS, Seybold ME. Cluster headache. Local anesthetic abortive agents. Arch Neurol 1985;42:496-8. [Crossref] [PubMed]
- Berger JJ, Pyles ST, Saga-Rumley SA. Does topical anesthesia of the sphenopalatine ganglion with cocaine or lidocaine relieve low back pain? Anesth Analg 1986;65:700-2. [Crossref] [PubMed]
- Yang lY, Oraee S. A novel approach to transnasal sphenopalatine ganglion injection. Pain Physician 2006;9:131-4. [PubMed]
- Mehta D, Leary MC, Yacoub HA, et al. The Effect of Regional Anesthetic Sphenopalatine Ganglion Block on Self-Reported Pain in Patients With Status Migrainosus. Headache 2019;59:69-76. [Crossref] [PubMed]
- Piagkou M, Demesticha T, Troupis T, et al. The pterygopalatine ganglion and its role in various pain syndromes: from anatomy to clinical practice. Pain Pract 2012;12:399-412. [Crossref] [PubMed]
- Vallejo R, Benyamin R, Yousuf N, et al. Computed tomography-enhanced sphenopalatine ganglion blockade. Pain Pract 2007;7:44-6. [Crossref] [PubMed]
- Cheng J, Abdi S. Complications of joint, tendon, and muscle injections. Tech Reg Anesth Pain Manag 2007;11:141-7. [Crossref] [PubMed]
- Sanders M, Zuurmond WW. Efficacy of sphenopalatine ganglion blockade in 66 patients suffering from cluster headache: a 12- to 70-month follow-up evaluation. J Neurosurg 1997;87:876-80. [Crossref] [PubMed]
- Narouze S, Kapural L, Casanova J, et al. Sphenopalatine ganglion radiofrequency ablation for the management of chronic cluster headache. Headache 2009;49:571-7. [Crossref] [PubMed]
- Narouze SN. Role of sphenopalatine ganglion neuroablation in the management of cluster headache. Curr Pain Headache Rep 2010;14:160-3. [Crossref] [PubMed]
- Standring SSS. Gray's anatomy: the anatomical basis of clinical practice. Elsevier Health Sciences; 41st International edition; 25 Sept. 2015:2016.
- Elias M. Cervical sympathetic and stellate ganglion blocks. Pain Physician 2000;3:294-304. [Crossref] [PubMed]
- Carron H, Litwiller R. Stellate ganglion block. Anesth Analg 1975;54:567-70. [Crossref] [PubMed]
- Vallejo R, Plancarte R, Benyamin RM, et al. Anterior cervical approach for stellate ganglion and T2 to T3 sympathetic blocks: a novel technique. Pain Pract 2005;5:244-8. [Crossref] [PubMed]
- Sinofsky A, Sharma T, Wright T. Stellate Ganglion Block for Debilitating Photophobia Secondary to Trigeminal, Postherpetic Neuralgia. Pain Pract 2016;16:E99-E102. [Crossref] [PubMed]
- Jeon Y. Therapeutic potential of stellate ganglion block in orofacial pain: a mini review. J Dent Anesth Pain Med 2016;16:159-63. [Crossref] [PubMed]
- Baron R, Jänig W, With H. Sympathetic and afferent neurones projecting into forelimb and trunk nerves and the anatomical organization of the thoracic sympathetic outflow of the rat. J Auton Nerv Syst 1995;53:205-14. [Crossref] [PubMed]
- Snell RS. Clinical anatomy for medical students. Boston: Little, Brown; 1995.
- Jeon Y, Kim D. The effect of stellate ganglion block on the atypical facial pain. J Dent Anesth Pain Med 2015;15:35-7. [Crossref] [PubMed]
- Wang QX, Wang XY, Fu NA, et al. Stellate ganglion block inhibits formalin-induced nociceptive responses: mechanism of action. Eur J Anaesthesiol 2005;22:913-8. [Crossref] [PubMed]
- Salvaggio I, Adducci E, Dell'Aquila L, et al. Facial pain: a possible therapy with stellate ganglion block. Pain Med 2008;9:958-62. [Crossref] [PubMed]
- Jeong S, Jeon Y, Yeo J, et al. The effects of stellate ganglion block on the electroencephalogram in rats. J Anesth 2014;28:601-5. [Crossref] [PubMed]
- Yeo J, Jeon Y. Effects of stellate ganglion block on sedation as assessed by bispectral index in normal healthy volunteers. Pain Physician 2015;18:173-8. [PubMed]
- Melis M, Zawawi K, al-Badawi E, et al. Complex regional pain syndrome in the head and neck: a review of the literature. J Orofac Pain 2002;16:93-104. [PubMed]
- Moon S, Lee J, Jeon Y. Bilateral stellate ganglion block for migraine: A case report. Medicine (Baltimore) 2020;99:e20023. [Crossref] [PubMed]
- Gupta PK, Gupta PK, Mahto SK, et al. Complex regional pain syndrome-A forgotten entity. J Family Med Prim Care 2019;8:1778-80. [Crossref] [PubMed]
- Heir GM, Nasri-Heir C, Thomas D, et al. Complex regional pain syndrome following trigeminal nerve injury: report of 2 cases. Oral Surg Oral Med Oral Pathol Oral Radiol 2012;114:733-9. [Crossref] [PubMed]
- Giri S, Nixdorf D. Sympathetically maintained pain presenting first as temporomandibular disorder, then as parotid dysfunction. J Can Dent Assoc 2007;73:163-7. [PubMed]
- Sago T, Takahashi O, Ogawa M, et al. Effects of stellate ganglion block on postoperative trigeminal neuropathy after dental surgery: a propensity score matching analysis. Sci Rep 2020;10:13463. [Crossref] [PubMed]
- Kim HJ, Ahn HS, Lee JY, et al. Effects of applying nerve blocks to prevent postherpetic neuralgia in patients with acute herpes zoster: a systematic review and meta-analysis. Korean J Pain 2017;30:3-17. [Crossref] [PubMed]
- Datta R, Agrawal J, Sharma A, et al. A study of the efficacy of stellate ganglion blocks in complex regional pain syndromes of the upper body. J Anaesthesiol Clin Pharmacol 2017;33:534-40. [Crossref] [PubMed]
- Ali NM. Does sympathetic ganglionic block prevent postherpetic neuralgia? Literature review. Reg Anesth 1995;20:227-33. [PubMed]
- Currey TA, Dalsania J. Treatment for herpes zoster ophthalmicus: stellate ganglion block as a treatment for acute pain and prevention of postherpetic neuralgia. Ann Ophthalmol 1991;23:188-9. [PubMed]
- Ueshima H. A successful case of stellate ganglion block for difficult therapy of refractory tension headache. J Clin Anesth 2019;54:149. [Crossref] [PubMed]
- Hentschel K, Capobianco DJ, Dodick DW. Facial pain. Neurologist 2005;11:244-9. [Crossref] [PubMed]
- Shanthanna H. Utility of stellate ganglion block in atypical facial pain: a case report and consideration of its possible mechanisms. Case Rep Med 2013;2013:293826. [Crossref] [PubMed]
- Totoki T, Yuda Y, Isa T, et al. Case of temporal arteritis. Masui 1973;22:480-4. [PubMed]
- Haraldson T, Mejersjö C. Temporal arteritis: a report on two cases. Swed Dent J 1982;6:121-5. [PubMed]
- Walega DR, Smith C, Epstein JB. Bilateral stellate ganglion blockade for recalcitrant oral pain from Burning Mouth Syndrome: a case report. J Oral Facial Pain Headache 2014;28:171-5. [Crossref] [PubMed]
- Piraccini E, Munakomi S, Chang KV. Stellate Ganglion Blocks. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- McLean B. Safety and Patient Acceptability of Stellate Ganglion Blockade as a Treatment Adjunct for Combat-Related Post-Traumatic Stress Disorder: A Quality Assurance Initiative. Cureus 2015;7:e320. [Crossref] [PubMed]
- WALLS WK. The anatomical approach in stellate ganglion injection. Br J Anaesth 1955;27:616-21. [Crossref] [PubMed]
- Hogan QH, Erickson SJ, Haddox JD, et al. The spread of solutions during stellate ganglion block. Reg Anesth 1992;17:78-83. [PubMed]
- Abdi S, Zhou Y, Patel N, et al. A new and easy technique to block the stellate ganglion. Pain Physician 2004;7:327-31. [Crossref] [PubMed]
- Wie C, Gupta R, Maloney J, et al. Interventional Modalities to Treat Complex Regional Pain Syndrome. Curr Pain Headache Rep 2021;25:10. [Crossref] [PubMed]
- Wang D. Image Guidance Technologies for Interventional Pain Procedures: Ultrasound, Fluoroscopy, and CT. Curr Pain Headache Rep 2018;22:6. [Crossref] [PubMed]
- Ghai A, Kaushik T, Wadhera R, et al. Stellate ganglion blockade-techniques and modalities. Acta Anaesthesiol Belg 2016;67:1-5. [PubMed]
- Narouze S. Ultrasound-guided stellate ganglion block: safety and efficacy. Curr Pain Headache Rep 2014;18:424. [Crossref] [PubMed]
- Wei K, Feldmann RE Jr, Brascher AK, et al. Ultrasound-guided stellate ganglion blocks combined with pharmacological and occupational therapy in Complex Regional Pain Syndrome (CRPS): a pilot case series ad interim. Pain Med 2014;15:2120-7. [Crossref] [PubMed]
- Lee MH, Kim KY, Song JH, et al. Minimal volume of local anesthetic required for an ultrasound-guided SGB. Pain Med 2012;13:1381-8. [Crossref] [PubMed]
- Goel V, Patwardhan AM, Ibrahim M, et al. Complications associated with stellate ganglion nerve block: a systematic review. Reg Anesth Pain Med 2019; Epub ahead of print. [Crossref] [PubMed]
- Narouze S, Vydyanathan A, Patel N. Ultrasound-guided stellate ganglion block successfully prevented esophageal puncture. Pain Physician 2007;10:747-52. [Crossref] [PubMed]
- Okuda Y, Kitajima T, Asai T. Stellate ganglion block, cervical sympathetic block and cervicothoracic sympathetic block. Eur J Anaesthesiol 1999;16:272-3. [Crossref] [PubMed]
- Huntoon MA. The vertebral artery is unlikely to be the sole source of vascular complications occurring during stellate ganglion block. Pain Pract 2010;10:25-30. [Crossref] [PubMed]
- Mishio M, Matsumoto T, Okuda Y, et al. Delayed severe airway obstruction due to hematoma following stellate ganglion block. Reg Anesth Pain Med 1998;23:516-9. [Crossref] [PubMed]
- ADRIANI J. PARMLEY J, OCHSNER A. Fatalities and complications after attempts at stellate ganglion block. Surgery 1952;32:615-9. [PubMed]
- Okuda Y, Urabe K, Kitajima T. Retropharyngeal or cervicomediastinal haematomas following stellate ganglion block. Eur J Anaesthesiol 2003;20:757-9. [Crossref] [PubMed]
- Gadient PM, Smith JH. The neuralgias: diagnosis and management. Curr Neurol Neurosci Rep 2014;14:459. [Crossref] [PubMed]
- Haładaj R, Polguj M, Topol M. Anatomical Variations of the Supraorbital and Supratrochlear Nerves: Their Intraorbital Course and Relation to the Supraorbital Margin. Med Sci Monit 2019;25:5201-10. [Crossref] [PubMed]
- Janis JE, Hatef DA, Hagan R, et al. Anatomy of the supratrochlear nerve: implications for the surgical treatment of migraine headaches. Plast Reconstr Surg 2013;131:743-50. [Crossref] [PubMed]
- Gil YC, Shin KJ, Lee SH, et al. Topography of the supraorbital nerve with reference to the lacrimal caruncle: danger zone for direct browplasty. Br J Ophthalmol 2017;101:940-5. [Crossref] [PubMed]
- Knize DM. A study of the supraorbital nerve. Plast Reconstr Surg 1995;96:564-9. [Crossref] [PubMed]
- Goldberg SW, Nahas SJ. Supratrochlear and Supraorbital Nerve Stimulation for Chronic Headache: a Review. Curr Pain Headache Rep 2015;19:26. [Crossref] [PubMed]
- Amin S, Buvanendran A, Park KS, et al. Peripheral nerve stimulator for the treatment of supraorbital neuralgia: a retrospective case series. Cephalalgia 2008;28:355-9. [Crossref] [PubMed]
- Dimitriou V, Iatrou C, Malefaki A, et al. Blockade of branches of the ophthalmic nerve in the management of acute attack of migraine. Middle East J Anaesthesiol 2002;16:499-504. [PubMed]
- Iljazi A, Chua A, Rich-Fiondella R, et al. Unrecognized challenges of treating status migrainosus: An observational study. Cephalalgia 2020;40:818-27. [Crossref] [PubMed]
- Waldman SD. Atlas of pain management injection techniques. Fourth edition. ed. St. Louis, Missouri: Elsevier; 2016.
- Tepe N, Tertemiz OF. Comparison of greater occipital nerve and greater occipital nerve + supraorbital nerve block effect in chronic medication overuse headache Turk J Med Sci 2021;51:1065-70. [Crossref] [PubMed]
- Johnson AP, Boscoe E, Cabrera-Muffly C. Local Blocks and Regional Anesthesia in the Head and Neck. Otolaryngol Clin North Am 2020;53:739-51. [Crossref] [PubMed]
- Novitch M, Hyatali FS, Jeha G, et al. Regional techniques for head and neck surgical procedures. Best Pract Res Clin Anaesthesiol 2019;33:377-86. [Crossref] [PubMed]
- Napier A, De Jesus O, Taylor A. Supraorbital Nerve Block. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Ilhan Alp S, Alp R. Supraorbital and infraorbital nerve blockade in migraine patients: results of 6-month clinical follow-up. Eur Rev Med Pharmacol Sci 2013;17:1778-81. [PubMed]
- Serra-Guillen C, Hueso L, Nagore E, et al. Comparative study between cold air analgesia and supraorbital and supratrochlear nerve block for the management of pain during photodynamic therapy for actinic keratoses of the frontotemporal zone. Br J Dermatol 2009;161:353-6. [Crossref] [PubMed]
- Yaghoubian JM, Aminpour S, Anilus V. Supertrochlear Nerve Block. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Latham JL, Martin SN. Infiltrative anesthesia in office practice. Am Fam Physician 2014;89:956-62. [PubMed]
- Liu W, Yang X, Li C, et al. Adverse drug reactions to local anesthetics: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol 2013;115:319-27. [Crossref] [PubMed]
- Nath S, Häggmark S, Johansson G, et al. Differential depressant and electrophysiologic cardiotoxicity of local anesthetics: an experimental study with special reference to lidocaine and bupivacaine. Anesth Analg 1986;65:1263-70. [Crossref] [PubMed]
- Yamanaka D, Kawano T, Shigematsu-Locatelli M, et al. Peripheral nerve block with a high concentration of tetracaine dissolved in bupivacaine for intractable post-herpetic itch: a case report. JA Clin Rep 2016;2:43. [Crossref] [PubMed]
- Pal GP, Routal RV, Saggu SK. The orientation of the articular facets of the zygapophyseal joints at the cervical and upper thoracic region. J Anat 2001;198:431-41. [Crossref] [PubMed]
- Yoganandan N, Knowles SA, Maiman DJ, et al. Anatomic study of the morphology of human cervical facet joint. Spine (Phila Pa 1976) 2003;28:2317-23. [Crossref] [PubMed]
- Bogduk N. The clinical anatomy of the cervical dorsal rami. Spine (Phila Pa 1976) 1982;7:319-30. [Crossref] [PubMed]
- Lord SM, Barnsley L, Bogduk N. Percutaneous radiofrequency neurotomy in the treatment of cervical zygapophysial joint pain: a caution. Neurosurgery 1995;36:732-9. [Crossref] [PubMed]
- Le DT, Alem N. Facet Joint Injection. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Peh W. Image-guided facet joint injection. Biomed Imaging Interv J 2011;7:e4. [PubMed]
- Kim BR, Lee JW, Lee E, et al. Intra-articular facet joint steroid injection-related adverse events encountered during 11,980 procedures. Eur Radiol 2020;30:1507-16. [Crossref] [PubMed]
- Hove B, Gyldensted C. Cervical analgesic facet joint arthrography. Neuroradiology 1990;32:456-9. [Crossref] [PubMed]
- Barnsley L, Bogduk N. Medial branch blocks are specific for the diagnosis of cervical zygapophyseal joint pain. Reg Anesth 1993;18:343-50. [PubMed]
- Barnsley L, Lord S, Bogduk N. Comparative local anaesthetic blocks in the diagnosis of cervical zygapophysial joint pain. Pain 1993;55:99-106. [Crossref] [PubMed]
- Bykowski JL, Wong WH. Role of facet joints in spine pain and image-guided treatment: a review. AJNR Am J Neuroradiol 2012;33:1419-26. [Crossref] [PubMed]
- Won HS, Yang M, Kim YD. Facet joint injections for management of low back pain: a clinically focused review. Anesth Pain Med (Seoul) 2020;15:8-18. [Crossref] [PubMed]
- Kershen LM, Nacey NC, Patrie JT, et al. Fluoroscopically Guided Facet Injections: Comparison of Intra-Articular and Periarticular Steroid and Anesthetic Injection on Immediate and Short-Term Pain Relief. AJNR Am J Neuroradiol 2018;39:2161-5. [Crossref] [PubMed]
- Ballestero MFM, Carneiro V, Luz Lima JP, et al. Psoas Abscess After Lumbar Facet Joint Injection: Case Report and Literature Review. Cureus 2020;12:e9559. [Crossref] [PubMed]
- Plastaras C, McCormick Z, Macron D, et al. Adverse events associated with fluoroscopically guided zygapophyseal joint injections. Pain Physician 2014;17:297-304. [PubMed]
- Ramos JA. Spinal injection of local anesthetic during cervical facet joint injection. Rev Bras Anestesiol 2016;66:654-6. [Crossref] [PubMed]
- Moore PA, Hersh EV. Local anesthetics: pharmacology and toxicity. Dent Clin North Am 2010;54:587-99. [Crossref] [PubMed]
- Blanton PL, Jeske AHADA Council on Scientific Affairs, et al. Avoiding complications in local anesthesia induction: anatomical considerations. J Am Dent Assoc 2003;134:888-93. [Crossref] [PubMed]
- Cummings DR, Yamashita DD, McAndrews JP. Complications of local anesthesia used in oral and maxillofacial surgery. Oral Maxillofac Surg Clin North Am 2011;23:369-77. [Crossref] [PubMed]
- Dickerson DM, Apfelbaum JL. Local anesthetic systemic toxicity. Aesthet Surg J 2014;34:1111-9. [Crossref] [PubMed]
- Wadlund DL. Local Anesthetic Systemic Toxicity. AORN J 2017;106:367-77. [Crossref] [PubMed]
- Ho JTF, van Riet TCT, Afrian Y, et al. Adverse effects following dental local anesthesia: a literature review. J Dent Anesth Pain Med 2021;21:507-25. [Crossref] [PubMed]
- Anitescu M, Benzon HT, Wallace MS. Challenging cases and complication management in pain medicine. Cham, Switzerland: Springer; 2018. doi:
10.1007/978-3-319-60072-7 .10.1007/978-3-319-60072-7 - Jespersen MS, Jaeger P, Ægidius KL, et al. Sphenopalatine ganglion block for the treatment of postdural puncture headache: a randomised, blinded, clinical trial. Br J Anaesth 2020;124:739-47. [Crossref] [PubMed]
- Fernandes L, Randall M, Idrovo L. Peripheral nerve blocks for headache disorders. Pract Neurol 2020; [Crossref] [PubMed]
Cite this article as: Thomas DC, Chablani D, Parekh S, Pitchumani PK, Mallareddy SD, Mathai BC. Nerve and ganglion blocks in the management of headache disorders: a narrative review. J Oral Maxillofac Anesth 2022;1:29.




