
Identifying and reducing risks of postoperative pulmonary complications
Background
Postoperative pulmonary complications (PPCs) are more common than postoperative cardiac events, but universal guidelines about prevention and patient optimization are lacking in this field. A systematic review found that PPCs occur in roughly one in eight patients undergoing surgery, which leads to over two-thirds of inpatient postoperative deaths (1). Extrapolation of data from more than 400 hospitals shows that over 1 million PPCs and nearly 50,000 deaths occur annually across the United States (US) due to development of respiratory complications after surgery (1). Multiple studies found that development of a PPC, including ones considered mild, was significantly associated with increased early postoperative mortality, intensive care unit (ICU) admission, and additional length of stay (LOS) in the ICU and hospital (1-3). Due to inconsistent definitions and variation in reporting, the rate of PPCs may be as high as 20% in certain patient populations depending on the diagnostic criteria used (1). Long-term outcomes can also be negatively impacted. Shander et al found that patients who experienced postoperative pneumonia, unplanned intubation, or failure to wean, the median survival decreased from 17.1 to 2.2 years (1).
PPCs pose a significant economic burden on patients and the healthcare system. Hospital LOS is a standard metric in determining the cost of service for inpatients. Overall, the mean increase in hospital LOS in patients who develop a PPC is an additional 8 days (2). One study found that a PPC occurring in patients undergoing noncardiac surgery led to an increased hospital stay of 89% and an additional hospital cost of 55% (1). Another study found a PPC in patients undergoing abdominal surgery increased the hospital LOS by 11 days, which accrued additional hospital charges of $31,000 (in year 2,000 US dollars) (1). When data was extrapolated from the entire US population, PPCs lead to an almost six figure amount of additional ICU admissions and over 3.4 billion dollars of additional costs (1). Since there is a significantly increased risk of patients being discharged to a skilled nursing facility rather than to their homes and families (1), there is the potential for an additional socioeconomic and mental health impact on this population.
Definitions
There are no universally accepted definitions of PPCs. In an attempt to improve standardization across clinical trials, European Perioperative Clinical Outcome (EPCO) definitions were created (4): respiratory infection, respiratory failure, pleural effusion, atelectasis, pneumothorax, bronchospasm, aspiration pneumonitis.
Pre-operative evaluation
Preoperative evaluations provide an opportunity to identify at risk patients or high-risk surgical factors, which can help guide patient optimization before going to the operating room; or the option of exploring non-surgical treatment options. Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria are used primarily by pulmonologists to standardize how to quantify the severity of chronic obstructive pulmonary disease (COPD). To identify patients at increased risk of PPCs, perioperative physicians tend to use the following risk assessment tools: ARISCAT score, GUPTA Postoperative Respiratory Failure Risk, GUPTA Postoperative Pneumonia Risk, and the Arozullah Respiratory Failure Index. However, what each means for the individual patient is still debated and the role of these risk indices may be better suited for comparing study populations. Most importantly, a thorough history and physical exam should be performed to evaluate patients for both known and unknown pulmonary comorbidities. This should include, exercise tolerance, symptoms of dyspnea or chest pain, history of recent cough or symptoms of respiratory tract infections; cardiac and pulmonary examination including auscultation. A recent (<1 month) history of respiratory tract infection can decrease pulmonary function, increase airway reactivity, and impair postoperative immunity (3). Although previously believed to have positive predictive value, new research shows pulmonary function tests (PFTs) are unnecessary, costly, and inconvenient for patients with known or suspected COPD. This data argues that a typical preoperative assessment which identifies medical conditions such as known COPD, the type of surgery, and relevant demographic information, is adequate to predict PPC events. Postponing surgery in order to obtain PFTs can lead to unnecessary costs and avoidable poor healthcare outcomes associated with patients hospitalized with nonproductive preoperative stay (5). One trial found that standardizing the preoperative assessment and staff education was pivotal for each patient to receive optimal care. The organization’s protocol; and educating patients and their families is essential in helping patients from prehabilitation to postoperative treatment across multiple health care providers (6).
Risk factors
A number of both patient-related and surgical-related risk factors have been identified that are predictive of PPCs. Surgical risk factors are more predictive and carry higher odds ratios of events.
Procedure-related risk factors include anatomical site (intrathoracic and upper abdominal incisions), duration of surgery longer than 2 hours, emergency surgery, and type of neuromuscular blockade and reversal agents used (2,3,7,8). Although the anatomical site of surgery is a nonmodifiable risk factor, the duration of surgery and emergent surgeries to some extent can be controlled by surgeons, as can rates of minimally invasive surgery (3). According to the Arozullah Respiratory Failure Index, orofacial surgery is one of the independent risk factors that confers the greatest risk of postoperative respiratory failure leading to prolonged ventilation or unplanned reintubation (7).
Newly published 2023 American Society of Anesthesiologists (ASA) Guidelines on Monitoring and Antagonism of Neuromuscular Blockade provide updated insights on avoiding residual paralysis in the post-anesthesia care unit (PACU). Inadequate reversal of neuromuscular blocking agents is strongly correlated with adverse outcomes such as atelectasis, upper airway obstruction, pneumonia, and reintubation (8). Incidence of residual neuromuscular blockade is as high as 64% of patients in the PACU. One contributing factor to this is a continued use of “clinical” assessments to check for paralysis, including sustained head lift and grip strength. As qualitative and quantitative tools to measure depth of paralysis become more widely available and affordable there is little reason to avoid such measures which can drastically improve patient outcomes (8). The new guidelines state when rocuronium and vecuronium are used, sugammadex is recommended over neostigmine for reversal from “deep, moderate, and shallow levels of neuromuscular blockade”. The concern about the current cost of sugammadex is offset by the decreased time to recovery, decreased residual neuromuscular blockade, as well as the costs associated with adverse events caused by residual paralysis (8).
Patient related risk factors include advanced age, COPD, poorly controlled asthma, heart failure, poor systemic health status defined by patients with an ASA classification of 2 or higher, cigarette smoking within 8 weeks of surgery, preoperative anemia (hemoglobin concentration lower than 10 g/dL), low preoperative peripheral oxygen saturation (SpO2), functional dependence, patients with current respiratory tract infections, and obstructive sleep apnea (OSA) (2,3,7). Advanced age was found to be one of the most predictive measures of PPCs. There is increased risk in those above 60; and considerably increased risk in those older than 80, even if otherwise healthy. This risk factor is becoming more relevant as developed countries have an aging population and more surgeries are being performed in this population which may have been avoided or forgone for other treatment options in the past (3). One large, multicenter study found the strongest patient related predictor of PPCs was the preoperative SpO2 while a patient was supine and breathing room air (3). Low preoperative oxygen saturations of less than 96% were weakly associated with increased risk of PPCs while a SpO2 of less than 90% was strongly associated with increased complications. Oxygen saturation is an easily recorded objective measure, which provides an opportunity for improvement. Measuring the SpO2 at the preoperative visit can help to identify high risk patients before surgery to help tailor the preoperative optimization and anesthetic plan.
Prevention/interventions
Multiple interventions have been identified in order to reduce PPCs following noncardiac surgery. These strategies include both optimizing the patient’s respiratory health in the preoperative period; as well as interventions in the intraoperative and postoperative periods to minimize the negative effects of surgery; anesthesia and immobility.
Current preoperative strategies include following enhanced recovery pathways, prophylactic mucolytics, prophylactic respiratory physiotherapy, oral hygiene, and smoking cessation of at least 4 weeks before surgery (9,10). Patients with COPD should be optimized to be at their baseline level of function, treating obstruction with medications such as inhaled bronchodilators, long-acting muscarinic antagonists (LAMA) and/or inhaled corticosteroids (5). Optimizing patients with poorly controlled asthma is crucial to minimizing PPCs in this patient population. After nearly 50 years where an inhaled short-acting beta agonist (SABA) has been the first line treatment for asthma, new guidelines from the Global Initiative for Asthma (GINA) Strategy states that a low-dose inhaled corticosteroid combined with a long-acting beta agonist (LABA) should be the new controller medication of choice (11). Oral hygiene should include brushing twice daily and daily use of mouthwash such as chlorhexidine, which can help to reduce the oral bacterial burden.
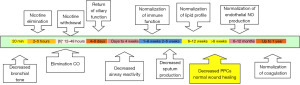
It is well known that smoking leads to a host of systemic problems as well as surgical complications. However, many preoperative providers do not provide smoking cessation interventions as part of their standard practice. Smoking cessation is a modifiable risk factor that can improve not only long-term patient outcomes, but short-term improvements before and after surgery. The Society for Perioperative Assessment and Quality Improvement (SPAQI) consensus statement declares that smoking cessation at any time, including both before and after surgery, incurs no risk of increased complication. Smoking cessation for as little as 4 weeks led to fewer respiratory and wound healing complications. Although smoking cessation for greater than 4 weeks is ideal, smoking cessation at any time is advantageous. For example, reduction of carbon monoxide levels (t1/2 1–8 hours) occurs in as little as 24 hours. See Figure 1 for a timeline of the many benefits of smoking cessation. In order to provide the largest window of abstinence, education of primary care physicians to provide smoking cessation before or at the time of surgical referral could lead to better outcomes for this patient population (10).
Intraoperative lung protective ventilation and goal directed fluid therapy has been shown to reduce the risk of PPCs (9). Other trials found that use of colloids, especially albumin, was an independent risk factor associated with development of PPCs. This is consistent with previous data that large fluid administration leads to poor surgical outcomes compared to goal directed fluid therapy, which has been adopted into enhanced recovery pathways (2). Lung protective ventilation involves the use of low tidal volumes (6–8 mL per kilogram of predicted body weight), higher levels of positive end-expiratory pressure (PEEP) to help prevent loss of alveoli surface area, and use of alveolar recruitment maneuvers. As discussed above, ensuring adequate reversal of neuromuscular blockade using quantitative or qualitative monitoring is essential to improving patient outcomes and reducing the risk of PPCs (8). Other than inadequate reversal of neuromuscular blockade, there is no strong evidence to show better outcomes in regional vs. general anesthetics. The type of anesthesia should be individualized for the specific patient. Since longer durations of surgery has been shown to increase the risk of PPCs (2,7,9), exploring different surgical interventions or other therapies should be discussed for patients deemed to be at a high risk for complications.
Postoperative strategies to reduce PPC burden include noninvasive ventilatory support, lung expansion maneuvers, early mobilization, adequate pain control, avoiding routine use of nasogastric tubes, following an enhanced recovery pathway, and appropriate monitoring (6,7,9,12). Noninvasive ventilatory support such as the use of continuous positive airway pressure (CPAP) can help stent open airways and decrease work of breathing (7,9). Other noninvasive ventilatory support options for those not established on CPAP include auto-titrating positive airway pressure (APAP) and high-flow nasal cannulae. Lung expansion techniques including chest physiotherapy, incentive spirometry, deep breathing exercises and CPAP have been proven to reduce PPC burden after abdominal surgery (12). Inadequate postoperative analgesia can lead to PPCs by preventing early ambulation and hindering the capacity to take deep breaths. Two studies found that fewer respiratory complications occurred in patient-controlled opioid analgesia groups (12). Incorporation of a multimodal analgesia strategy including non-opioid medications can help reduce the risk of PPCs (6,13). Nasogastric decompression should only be used for select indications, including reduction of postoperative nausea and vomiting (PONV), oral intake intolerance, and symptomatic abdominal distension. When used for these indications following abdominal surgery, it can help improve the return of baseline bowel function and reduce PPCs (12). One pulmonary care program that has incorporated these principles into practice is the I COUGH program. The acronym I COUGH designates the principles of Incentive spirometry, Coughing and deep breathing, Oral care (brushing teeth and using mouthwash twice daily), Understanding (patient and family education), Getting out of bed at least three times daily, and Head-of-bed elevation. After implementation there was a significant reduction in both postoperative pneumonia and unplanned reintubation. The multidisciplinary team who created the program stressed that education throughout the perioperative and postoperative period of the patient, family, nursing, and physician staff was vital to success of this initiative (6).
Identification and rescue
In the immediate postoperative period, respiratory complications remain the most common major problem. Failure to rescue leads to poor outcomes so monitoring is key in identifying issues that can be treated before deterioration. Upon arrival to PACU, ASA guidelines for post-anesthetic care include assessment of airway patency, respiratory rate, and SpO2. Frequent re-assessment of airway patency and continuous monitoring of respiratory rate and oxygen saturation are required (14). Other parameters that are assessed which may affect the patient’s respiration include neuromuscular function, cardiovascular function, mental status, temperature, pain, and PONV (14). Potential signs of respiratory insufficiency include, but are not limited to, tachypnea defined as respiratory rate greater than 30, shallow breathing, labored breathing using accessory muscles, bradypnea with a respiratory rate less than 8, and SpO2 below 94% possibly accompanied by cyanosis. Hypoxemia and hypercarbia can lead to anxiety, confusion, somnolence, obtundation, and if left untreated these can result in malignant arrythmias or cardiac arrest.
While performing initial assessment of patients who have signs and symptoms of respiratory depression, stabilize the patient with supplemental oxygen via facemask or non-rebreather, noninvasive ventilatory support, and clearing any secretions or relieving any obstruction. Although a complete obstruction may be silent, signs of a partial obstruction may include snoring, labored breathing, or inspiratory stridor on auscultation. Lung auscultation can also help to diagnosis and differentiate between respiratory complications such as bronchospasm, upper versus lower airway obstructions, pulmonary edema, atelectasis, and aspiration. An arterial blood gas analysis can help to determine if there are abnormal oxygen and carbon dioxide values or electrolyte derangements present. A bedside chest radiograph also provides diagnostic value for many respiratory complications. Altered levels of consciousness may be a clue to hypercapnia or hypoxemia due to hypoventilation, which can be caused by opioid induced respiratory depression, residual neuromuscular blockade, or other neurologic deficits (8,13). Clinical findings will inform next steps of medication management: bronchodilators vs. diuretics vs. reversal of opioid or other depressant agents.
Vigilance needs to continue after the PACU stay, especially in patients identified as high risk for PPCs. The PRODIGY trial found that use of continuous capnography and pulse oximetry can be an invaluable tool to prevent opioid-induced respiratory depression episodes on the general care floors. With nearly half of all cardiorespiratory events occurring on the general care floors, continuous respiratory monitoring provides an opportunity to recognize abnormalities early and prevent adverse events to decrease morbidity and mortality (13). Appropriate response to abnormalities follow the aforementioned assessment and intervention protocols. The cost effectiveness and availability of continuous respiratory monitors have precluded its widespread use thus far.
Upon discharge, patients need to be educated to follow enhanced recovery pathways of early mobilization, proper oral hygiene, and continued smoking cessation. Patients should be encouraged to take ownership of their home care in order to facilitate their recovery once they leave the healthcare facility. One important factor to stress is continued smoking cessation in the postoperative period. Berman found after lumbar and cervical spinal fusions, smoking significantly increased the risk of pseudarthrosis, infection, dysphagia, and adjacent-segment pathology (15). They determined the highest recommendation they can provide is smoking cessation for the first 4 weeks after surgery because this is the most critical time period for angiogenesis (15). Another study by Twito et al. assessing the healing period after dental implant placement, recommends that smoking cessation should be extended to 8 weeks following surgery in order to complete the healing phase of osteoblasts and improve osseointegration (16).
Coronavirus disease 2019 (Covid-19)
Patients positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or Covid-19 infection should heavily influence the perioperative evaluation and timing of surgery. By itself, Covid-19 infection can lead to significant respiratory illness, multiorgan failure, and death. Patients infected with Covid-19 who underwent surgery had the highest rates of respiratory failure, pneumonia, pulmonary embolism, renal failure, and sepsis compared to control. Surgery performed 4 to 8 weeks after Covid-19 infection had an increased risk of pneumonia, even in patients with mild to moderate symptoms. Deng et al. found that it was not until the 8-week period after diagnosis where there was no longer an association with increased postoperative complications (17). A larger collaborative study out of Europe demonstrated no improvement in PPC rate until at least 7 weeks after initial Covid-19 diagnosis (17). Although more data is needed, it is recommended for patients undergoing elective surgery to wait at least 4 weeks after diagnosis of asymptomatic cases and 6–8 weeks for symptomatic patients. For the preoperative evaluation of these patients, it is recommended to do a full history and physical, determine if the patient has returned to their “pre-Covid” baseline, functional capacity assessment, and a frailty assessment in those over 65 or anyone who required hospitalization for treatment of symptoms (18). An article offering guidance early in the pandemic, when the virulent strain was dominant, suggested more extensive preoperative testing than is usually done. Significant abnormalities in these tests would warrant specialty discussion or consults and further testing such as an echocardiogram, coagulation tests, or a chest radiograph (18). Newer milder variants have changed this approach. Although more information is needed, there are significant concerns for postoperative complications arising in patients with “post-Covid syndrome” or “Long Covid.” One concern is the cognitive effect of long Covid and possible additive effect of surgery, anesthesia and admission in vulnerable patients.
Conclusions
PPCs occur more frequently than realized, and are associated with significant morbidity, mortality and cost. Surgical factors carry higher risk than patient factors. All have modifiable elements to them. Validated risk assessment tools are available and may provide individualized risk scores. Early (>4 weeks preoperatively) interventions and meticulous intra- and post-operative care can decrease the risk (Table 1). Monitoring and early rescue treatment is needed for when complications still occur despite best practice preparation and care.
Table 1
| Time | Strategies/interventions |
|---|---|
| Preoperative | Enhanced recovery pathways |
| Prophylactic mucolytics | |
| Prophylactic respiratory physiotherapy | |
| Oral hygiene | |
| Smoking cessation at least 4 weeks before surgery | |
| Optimization of COPD with medication | |
| Intraoperative | Lung protective ventilation |
| Goal directed fluid therapy | |
| Minimizing colloid use | |
| Adequate neuromuscular blockade reversal | |
| Postoperative | Noninvasive ventilatory support |
| Lung expansion maneuvers | |
| Early mobilization | |
| Adequate pain control | |
| Avoiding routine use of nasogastric tubes | |
| Enhanced recovery pathway | |
| Appropriate monitoring |
PPCs, postoperative pulmonary complications; COPD, chronic obstructive pulmonary disease.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://joma.amegroups.com/article/view/10.21037/joma-23-20/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://joma.amegroups.com/article/view/10.21037/joma-23-20/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shander A, Fleisher LA, Barie PS, et al. Clinical and economic burden of postoperative pulmonary complications: patient safety summit on definition, risk-reducing interventions, and preventive strategies. Crit Care Med 2011;39:2163-72. [Crossref] [PubMed]
- Fernandez-Bustamante A, Frendl G, Sprung J, et al. Postoperative Pulmonary Complications, Early Mortality, and Hospital Stay Following Noncardiothoracic Surgery: A Multicenter Study by the Perioperative Research Network Investigators. JAMA Surg 2017;152:157-66. [Crossref] [PubMed]
- Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010;113:1338-50. [Crossref] [PubMed]
- Jammer I, Wickboldt N, Sander M, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol 2015;32:88-105. [Crossref] [PubMed]
- Dankert A, Neumann-Schirmbeck B, Dohrmann T, et al. Preoperative Spirometry in Patients With Known or Suspected Chronic Obstructive Pulmonary Disease Undergoing Major Surgery: The Prospective Observational PREDICT Study. Anesth Analg 2023;137:806-18. [Crossref] [PubMed]
- Cassidy MR, Rosenkranz P, McCabe K, et al. I COUGH: reducing postoperative pulmonary complications with a multidisciplinary patient care program. JAMA Surg 2013;148:740-5. [Crossref] [PubMed]
- Smetana GW. Postoperative pulmonary complications: an update on risk assessment and reduction. Cleve Clin J Med 2009;76:S60-5. [Crossref] [PubMed]
- Thilen SR, Weigel WA, Todd MM, et al. 2023 American Society of Anesthesiologists Practice Guidelines for Monitoring and Antagonism of Neuromuscular Blockade: A Report by the American Society of Anesthesiologists Task Force on Neuromuscular Blockade. Anesthesiology 2023;138:13-41. [Crossref] [PubMed]
- Odor PM, Bampoe S, Gilhooly D, et al. Perioperative interventions for prevention of postoperative pulmonary complications: systematic review and meta-analysis. BMJ 2020;368:m540. [Crossref] [PubMed]
- Wong J, An D, Urman RD, et al. Society for Perioperative Assessment and Quality Improvement (SPAQI) Consensus Statement on Perioperative Smoking Cessation. Anesth Analg 2020;131:955-68. [Crossref] [PubMed]
- Reddel HK, Bacharier LB, Bateman ED, et al. Global Initiative for Asthma Strategy 2021: executive summary and rationale for key changes. Eur Respir J 2022;59:2102730. [Crossref] [PubMed]
- Lawrence VA, Cornell JE, Smetana GW, et al. Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med 2006;144:596-608. [Crossref] [PubMed]
- Khanna AK, Bergese SD, Jungquist CR, et al. Prediction of Opioid-Induced Respiratory Depression on Inpatient Wards Using Continuous Capnography and Oximetry: An International Prospective, Observational Trial. Anesth Analg 2020;131:1012-24. [Crossref] [PubMed]
- Apfelbaum JL, Silverstein JH, Chung FF, et al. Practice guidelines for postanesthetic care: an updated report by the American Society of Anesthesiologists Task Force on Postanesthetic Care. Anesthesiology 2013;118:291-307. [Crossref] [PubMed]
- Berman D, Oren JH, Bendo J, et al. The Effect of Smoking on Spinal Fusion. Int J Spine Surg 2017;11:29. [Crossref] [PubMed]
- Twito D, Sade P. The effect of cigarette smoking habits on the outcome of dental implant treatment. PeerJ 2014;2:e546. [Crossref] [PubMed]
- Deng JZ, Chan JS, Potter AL, et al. The Risk of Postoperative Complications After Major Elective Surgery in Active or Resolved COVID-19 in the United States. Ann Surg 2022;275:242-6. [Crossref] [PubMed]
- Bui N, Coetzer M, Schenning KJ, et al. Preparing previously COVID-19-positive patients for elective surgery: a framework for preoperative evaluation. Perioper Med (Lond) 2021;10:1. [Crossref] [PubMed]
Cite this article as: Sigona A, Richman DC. Identifying and reducing risks of postoperative pulmonary complications. J Oral Maxillofac Anesth 2023;2:30.


