
Oromandibular dystonia and temporomandibular disorders—a review on diagnosis and management
Introduction
Oromandibular dystonia (OMD) is characterized by sustained involuntary, repetitive and patterned contractions of muscles. It is a movement disorder that commonly involves masticatory, lingual, labial and lower facial muscles (1). The prevalence of OMD varies greatly depending on the race and ethnicity, approximately affecting 170 per 100,000 people. It often affects women more than man with an average onset age of 50–60 years (2).
OMD can be inherited, having a genetic basis, primary or idiopathic with no specific cause or secondary resulting from underlying disorders. Secondary dystonia can be caused by a range of conditions including neurodegenerative disorders, encephalitis, traumatic brain injuries, stroke, multiple sclerosis, metabolic disorders such as Wilson diseases and neuroleptic medications for example chlorpromazine, haloperidol, etc. (3). Peripheral tissue injury, including dental extractions, orofacial trauma from ill-fitting dentures has also been linked in certain accounts to the onset of OMD (2-5). Although the exact pathophysiology of OMD is still unknown, central mediated dysregulation of basal ganglia particularly in the sensorimotor regions of putamen are thought to be involved. Additionally genetic, impaired inhibitory process, abnormal sensory feedback, maladaptive neuroplasticity are involved (6).
According to the type of movement, OMD can be categorized as jaw opening dystonia, jaw closing, jaw deviation, jaw protrusion, lingual dystonia or a combination of these five movements (7). OMD can manifest in isolation or in conjunction with other types of focal dystonia such as blepharospasm commonly known as Meige syndrome, spasmodic dysphonia or cervical dystonia (4,8). OMD movements may also extend to extra-cranial regions such as the cervical region which is known as craniocervical dystonia. OMD is frequently triggered by movement execution which is exacerbated by emotional stress and daily activities as talking and chewing (2). However, some behaviors such as biting the lip, biting on a toothpick, touching tongue to palate swallowing, whistling, and sleeping can provide brief respite from OMD symptoms (8).
The commonly report sign and symptoms of OMD are persistent teeth clenching and grinding, fractured teeth and restorations, trauma to the lip and gingiva, masticatory disturbances, fatigue, muscle pain and joint pain, joint sounds, jaw locking, dysarthria, dysphagia and esthetic problems (9). Additionally, some patients described symptoms of aspiration pneumonia from lingual dystonia and upper airway obstruction from temporomandibular dislocation caused on by severe jaw opening dystonia (10). These symptoms can result in social embarrassment, cosmetic disfigurement and impact patient overall quality of life (11).
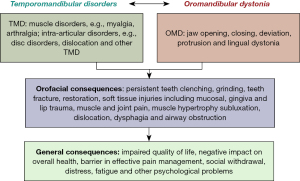
Patients with OMD often report clinical signs and symptoms of temporomandibular disorders (TMD) (Figure 1). The TMD are a cluster of musculoskeletal conditions that affect masticatory muscles and temporomandibular joint (TMJ) and/or associated structures (12). TMD is a multifactorial disease process caused by trauma either physical or emotional, biological process such as aging, systemic predisposition, sleep disorders and psychosocial alterations. The major symptoms include joint and muscle pain, joint noises and functional limitation or decreased mandibular range of motion. TMD can be broadly classified as articular disorders in which signs and symptoms are related to TMJ, and muscular disorders which manifest signs and symptoms related to stomatognathic musculature (12). The common TMD diagnosis observed in patients with OMD are myalgia, arthralgia, disc disorders, osteoarthritis, subluxation, luxation, dislocation of TMJ and headaches attributed to TMD (12). These disorders have high risk of psychiatric and psychological co-morbidity which leads to social withdrawal (13) and profoundly affect patients’ quality of life (14). Often, it is challenging for the clinicians to differentiate between the clinical characteristics of two conditions which may potentially result in misdiagnosis and incorrect management (14).
Gap of knowledge
Studies have investigated the prevalence and clinical characteristics of patients with OMD who had diagnosis of TMD (2,14). Nevertheless, there is a limited information available for practitioner in this area involved in delivering care to these patients. Therefore, understanding the intricacy of OMD in individuals with TMD diagnosis, and examining their similarities as well as the consequences associated with them will enable the clinician to address this combination.
Objective
The objective of this review is to provide clinicians with an enhanced understanding on the diagnosis of OMD and TMD, along with a brief discussion on common management strategies. Importantly, the review also covers the role of botulinum neurotoxins (BoNT) injections techniques to improve the quality of life for patients suffering from OMD.
Diagnosis of OMD and TMD
An interdisciplinary evaluation involving dental practitioner, oral surgeons, medical doctors or neurologists/movement disorder specialist, psychiatrist are needed for the diagnosis of OMD. OMD is frequently diagnosed clinically based on the clinical symptoms, signs and subtypes. For diagnosis of OMD a validated Oromandibular Dystonia Questionnaire (OMDQ-25) was developed (15). Recently, another OMD screening questionnaire was developed for the differential diagnosis of OMD from other disorders such as dyskinesia and functional movement disorders (16,17). However, still lack of clinical guidelines poses a significant challenge to the clinicians in early diagnosis (17).
The clinical evaluation of OMD begins with detailed history, with special focus on medication history and examination, subtype of muscle contractions (18). The history and examination should address if dystonia is the only problem or if there are other relevant problems as TMD that are a part of recognizable condition (19). The examination should be performed both with patient at rest (eyes open and closed) and while they are performing function. When a clinical diagnosis of dystonia is suspected based on clinical evaluation, the first step is to rule out other disorders that mimic dystonia such as neuromuscular and psychogenic disorders. Additionally, dystonia patients who experience dysphagia should have a swallowing and nutritional assessment to determine oropharyngeal function and nutrition status (20).
The age at which dystonia first appeared serves a reference for various etiologies. Adults are most likely to be idiopathic and children are more likely to have identifiable etiology. The progression of symptoms over time provides additional information on possible etiology for instance adult dystonia often progress from a week to a few years with successive evolution being slower and more difficult to recognize (21). Rapid evolution can indicate a psychogenic cause, a medication effect or vascular condition (19). Furthermore, it is important to know the involved region or type of dystonia since it provides guide to therapeutic approach. For instance, BoNT are used in cases of focal dystonia and alternative strategies such as oral medications, neurosurgical interventions and other approaches are used for generalized dystonia (19). Moreover, the effects of sensory tricks in alleviating the dystonic activities should be considered. Patient self-reported observations should not be neglected as certain patterns may go undetected during clinical examination (19,20).
Additional diagnostic testing is often needed to rule out any specific disorders. For instance, to rule out Wilson’s disease, laboratory investigations such as serum ceruloplasmin level and slit lamp exam should be performed for all patient who present in childhood or adolescence or early adulthood (19,20,22). Electromyography (EMG) often shows excessive muscle activity, but it is not commonly used because the findings are not dystonia specific.
Brain magnetic resonance imaging (MRI), magnetic resonance angiography and functional MRI should be performed to rule out central degenerative diseases, demyelinating or sclerotic lesion of the nervous system while genetic testing needed to rule out any specific inherited disorder (8,19,20,23) (Figure 2).

Since individuals with OMD experience TMD symptoms as a consequence of dystonic movements, an additional evaluation is sometimes warranted. For diagnosis of TMD, a descriptive history and clinical assessment should be given special attention. Additional diagnostic imaging may be required in some cases to render a definitive TMD pain diagnosis and rule out other pathology (24). The Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) include pain history questionnaires as well as validated clinical examination criteria for diagnosis of TMD (25). It also offers Axis II questionnaires for evaluating psycho-social and behavioral aspects that may have an impact on the onset and persistence of patient’s TMD. Recently, the International Classification of Orofacial Pain (ICOP) has provided a comprehensive description of pain conditions affecting orofacial region including TMD along with structured diagnostic criteria (26).
Management of OMD and TMD
Typically, mechanism-based treatments are optimal however, identifying the mechanism behind OMD and TMD is challenging. The objective of management for most of the individuals should be focused on improved quality of life, pain reduction and decrease the frequency and duration of abnormal movements (27). An appropriate management strategy should be tailored to alleviate the sign and symptoms of the condition. Ideally, a patient-centered or individualized management approach should be implemented considering characteristics of the individual such as comorbidities, medications, genetics and existing treatments (8,19).
Conservative treatments for TMD include education and reassurance, self-care, oral appliances, physical therapy and ergonomic modifications, therapeutic exercise, dry needling and acupuncture, stress management, and relaxation techniques when indicated (24). Other treatments in such a framework can include steroids, non-steroidal anti-inflammatory drugs, opioid and non-opioid pharmacological therapies, injectable pharmacotherapies, psychological therapies, integrative treatments, and non-surgical interventional and surgical procedures (24). For mild to moderate TMD, typically conservative treatment approach is preferred over surgery since it is less invasive and usually results in satisfactory clinical outcomes. While, according to the literature approximately 10–15% of all individuals undergoing treatment for TMD require surgical intervention. There is a spectrum of surgical procedures ranging from arthrocentesis and arthroscopy to more complex open joint surgical procedures arthrotomy or joint replacement (28).
Management of OMD often require multidisciplinary evaluation and involve different options such as counseling and education, psychological therapies, oral medications (benzodiazepine, zolpidem, tetrabenazine, cholinergics, baclofen, dopaminergic agents, anti-convulsants and lithium), intramuscular injections of BoNT, physical therapy, occupational therapy, oral appliances and neurosurgical interventions (20,29). Medications are prescribed in early stages and may have some effects in controlling dystonic movements. However, the evidence is still conflicting (19,20,29-31). Muscle afferent block using intramuscular injections of lidocaine and alcohol was proposed as a treatment for drug resistant OMD however the evidence is limited (8). There is emerging evidence for the use of deep brain stimulation of the subthalamic nucleus and globus pallidus interna in patients with refractory dystonia (32). Evidence-based reviews and clinical experience strongly suggests BoNT as first line of treatment for OMD regardless of its clinical presentation.
Role of botulinum toxin in OMD
Currently, intramuscular injections of BoNT are used to treat number of head and neck pain disorders especially when the problem is of muscle disorders. The Food and Drug Administration has approved BoNT injections for the certain movement disorders such as blepharospasm and cervical dystonia (33). Despite a paucity of studies supporting high grade evidence of its effectiveness, BoNT has been increasingly used to treat muscle spasticity, myofascial pain, bruxism, muscle hypertrophy, arthralgia and other intra-articular disorders (34). However, due to diversity of TMD diagnosis data on injection protocol, method, dose and site of injections is still lacking. Hence further studies are required to establish the effectiveness and standardization of BoNT in TMD.
BoNT injections are also considered to be most effective treatment for OMD in clinical practice. BoNT are synthetic derivatives of naturally occurring toxins produced by clostridium botulinum bacterium. This toxin inhibits the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex, which prevents acetylcholine from being released at the neuromuscular junction (35). Additionally, BoNT is effective in managing pain via its interaction with the SNARE complex that blocks synaptic vesicle fusion and inhibits the release of various neurotransmitters including glutamate, substance, calcitonin gene-related peptides and pain sensing trans membrane receptors such as transient receptor potential channels on neuronal membranes (36). Several stereotypes have been discovered and two of them serotype A and B have been developed as therapeutics. The serotype A include abobotulinum toxin A known as Dysport®, incobotulinum toxin A (XeominTM) and onabotulinum toxin A (Botox®) while serotype B available as rima botulinum toxin B (MyoblocTM). Other formulations are also available in specific parts of the world (8,37).
In OMD patients, BoNT reduce abnormal movements, pain, spasms and disability. Several systematic and evidence-based reviews have summarized the safety, efficacy, muscle selection and dosing of BoNT (38,39). Generally, doses of BoNT vary among different products but effects are similar. Amount of dose injected in the muscles is tailored to each patient. Benefits usually appear within a week and last for approximately 3 months (40).
In the past few years, American Academy of Neurology have provided the level of evidence for the treatment of OMD with BoNT. The abobotulinum toxin A and onabotulinum toxin A were given level C recommendation (possibly effective in the specified population) while incobotulinum toxin A and rima botulinum toxin B had a level U recommendation (data is inadequate and treatment is unproven) (8,27). Due to lack of evidence with Inco-BoNT and Rima-BoNT, we have discussed common injection techniques only with specific dosage of Ona-BoNT (Botox®) and Abo-BoNT (Dyport®) (Table 1).
Table 1
| Dystonia classification and common injected muscles | Muscle(s) involved and dosage of BoNT |
|---|---|
| (I) Types of oromandibular dystonia | |
| Movements | |
| Jaw opening dystonia | Lateral pterygoid, and digastric |
| Jaw closing dystonia | Masseter, temporalis, and medial pterygoid |
| Jaw deviating and protrusion dystonia | Lateral pterygoid, and medial pterygoid |
| Lingual dystonia | Genioglossus, superior and inferior longitudinal |
| Perioral dystonia | Orbicularis oris, zygomaticus, risorius, levator anguli oris, depressor anguli oris, mentalis, levator labii nasi and platysma |
| (II) BoNT dosage for each muscle | |
| Common muscles | |
| Masseter | 30 units of Ona-BoNT or 100 units of Abo-BoNT on each side |
| Temporalis | 20–30 units for Ona-BoNT or 60–80 units for Abo-BoNT on each side |
| Medial pterygoid | 20 units for Ona-BoNT or 30 units for Abo-BoNT on each side |
| Lateral pterygoid | 20–40 units for Ona-BoNT or 60 units for Abo-BoNT |
| Genioglossus (superior and inferior longitudinal muscles) | 10–20 units of Ona-BoNT or 30–50 units of Abo-BoNT |
| Mylohyoid | 20 units of Ona-BoNT or 90 units of Abo-BoNT |
| Platysma | 20 units of Ona-BoNT or 90 units of Abo-BoNT |
BoNT, botulinum neurotoxins.
Common injection techniques in OMD
In patients with OMD substantial benefits can be achieved by limiting the injections to the affect muscles only. Considering that oromandibular region consists of various muscle groups and vital structures, a targeted technique is often indicated to allow exact localization of affected muscles. Adjunct tools such as EMG and ultrasonography are not required for all the muscles but in certain muscles such as lateral pterygoid the use of targeting technique with EMG is recommended (8,38).
When administrating BoNT injections for the first time, particularly in patients who are not familiar with BoNT the general recommendation is to use the smallest effective initial dose (8). This helps to avoid unwanted side effects such as muscle weakness. Although there is a consensus in the starting dosage for each toxin, the maximum dose used in OMD patients varies. Subsequently, injection is individualized for each patient. Generally, BoNT injections are prepared with a 30-gauge needle for superficial muscles and a 27-gauge EMG needle for deeper muscles that are difficult to visualize, such as lateral pterygoids (27) (Figure 3).
Jaw closing dystonia
For jaw closing OMD generally, masseter, temporalis and medial pterygoid were selected for injections. The masseter can be approached at 1cm anterior to the posterior border of the ramus. Clenching of the teeth can aid in localization of the masseter muscles. The starting dose for masseter is 30 units of Ona-BoNT (Botox®) or 100 units of Abo-BoNT (Dyport®) on each side. Injection of parotid gland which overlies the posterior border of the masseter should be avoided (8). The temporalis muscle can be approached perpendicular to its plane and high as possible in the temporalis fossa due to wide radiating pattern of muscle fibers. Since the lower portion of the muscle is primarily made up of tendons and injections might be painful in this region, it is normally avoided (8). The dose for temporalis is 20–30 units for Ona-BoNT (Botox®) and 60–80 units for Abo-BoNT (Dyport®) on each side (39,40).
To avoid the risk of complications such as hematoma, the medial pterygoid injection often requires EMG guidance. It can be approached intraorally or extra-orally. For intra oral approach the muscle palpated at the inner aspect of the mandibular ramus with mouth open and a needle should be inserted at the medial aspect of the mandibular ramus angulated posterior and superior about 20 degrees to the occlusal plane respectively (27,41).
For extra-oral approach the needle is pointed below and inserted approximately 0.5–1 cm anterior to the angle of mandible along the interior aspect of the mandible and angled perpendicularly to the mandible. Precautions should be taken to avoid injury to facial artery. The dose for medial pterygoid is 20 units for Ona-BoNT (Botox®) and 30 units for Abo-BoNT (Dyport®) (8,39,41).
Jaw opening dystonia
Lateral pterygoid can be approached intraorally and laterally via mandibular notch and extraorally. For intra-oral approach patient were typically placed in a semi-reclined position with mouth slightly open and shift to the opposite side. Generally, needle is inserted above the mucobuccal fold above the second molar until it hit the lateral pterygoid plate and BoNT is injected. In some cases, the needle is also redirected superiorly to hit the infra-temporal crest of greater wing of sphenoid (42).
However, intraoral approach is challenging in patients with widely displaced mandibular movements. For extra oral approach under an EMG guidance the needle is angled upward at about 15 degrees to reach inferior head of the lateral pterygoid with an entry point about 3.5 cm from external auditory canal and 1 cm from the inferior margin of the zygomatic arch (7,8).
Care should be taken to avoid injury to pterygoid branch of the maxillary artery. The effective starting dose each side is 20–40 units for Ona-BoNT (Botox®) and 60 units for Abo-BoNT (Dyport®). In some cases, injections into the anterior digastric, geniohyoid, mylohyoid, submental muscles and platysma can provide additional benefits to the patients (27,43).
Jaw-deviating and jaw protrusion dystonia
The contralateral lateral pterygoid works in conjunction with the contralateral medial pterygoid to deviate the mouth from one side to the other, while lateral pterygoid helps in protrusion and temporalis pulls the jaw to the same side. The injections should follow the same techniques as discussed above.
Lingual dystonia
Lingual dystonia is a movement that clinician often encountered in OMD. It is divided into protrusion, retraction, laterotrusion and curling. Given that lingual muscles intermingle with each other the major muscles involved in dystonia are genioglossus, geniohyoid, hyoglossus and intrinsic muscles (superior and inferior longitudinal muscles). The genioglossus usually approached via submandibular region under EMG guidance through the digastric muscle with 2 cm in depth. The patient was placed in the supine position with the head tilted backwards. The injections are generally 2.5–3 cm posterior to the chin and about 1 cm on either side of middle and approximately 1.5–2 cm apart from each other. In some cases, additional injections into the superior and inferior longitudinal muscles to 5–10 mm depth are needed. The initial dose per side varies from 10–15 units of Ona-BoNT (Botox®) and 30–50 units of Abo-BoNT (Dyport®) respectively (8,27). Care should be taken as doses above the therapeutic level can cause disabling weakness associated with serious dysphagia, aspiration pneumonia and breathing difficulties (44).
Adverse effects
There are studies which support the fact that BoNT provides excellent benefits (37,45,46). However, in the meta-analysis, dysphagia is noted to be the most common adverse event (47). Other reported adverse effects are local hematoma, weakness in the muscles, hoarseness in voice, dysarthria, blurred vision, lower facial asymmetry, lip numbness and dry mouth due to diffusion of BoNT in the salivary glands and aspiration. Systemic symptoms are rare still there are cases documented having allergic reactions, flue like symptoms and non-responsiveness to BoNT due to immunoresistance (formation of antibodies that neutralize BoNT proteins) (37,47). Even after the decades of treatment there are still no deleterious effects, hence the choice of BoNT, injection techniques dosage largely depends on the experience and preferences of individual providers.
Strengths and limitations
This is a standard review that focuses on the clinical approach towards diagnosing and managing individuals with coexisting OMD and TMD. It also familiarizes the practitioners with comprehensive overview on different injections techniques, BoNT dosages, and special consideration to be mindful of when managing OMD. However, the present review has limitations. For example, literature reviews comparing the effectiveness of different treatment options in individuals with OMD and TMD were not documented. Furthermore, paucity of research and no consensus opinions on the diagnosis of management OMD was another limiting factor. Hence, a future collaborative multi centric studies with substantial number of OMD individuals with TMD diagnosis are warranted to establish new diagnostic strategies and effective treatment alternatives.
Conclusions
OMD is a common movement disorder that causes significant interference with functioning and impact patients’ quality of life. The abnormal movements and postures typically contribute to the symptoms of TMD. Hence, during patient evaluation, greater emphasis should be placed on patients’ signs and symptoms, as well as awareness of other differential diagnoses (Table 2). BoNT provides relief in majority of the patient, however additional treatment strategies should be considered on the individual basis. Finally, to obtain a satisfactory treatment effect, a multidisciplinary approach is warranted.
Table 2
| (I) A comprehensive history focuses on medications, exacerbating and relieving factors and subtype of muscle movements are important for diagnosing OMD |
| (II) Early screening and diagnosis of TMD in patients with OMD is likely to greatly improve the prognosis, quality of life and reduce health care costs |
| (III) It is essential for the providers to be familiar with clinical manifestations of OMD and TMD |
| (IV) Individuals diagnosed with OMD should undergo routine evaluation for TMD |
| (V) OMD can be managed successfully with combination of botulinum toxin injections, oral medications, physical therapy, oral appliances and deep brain stimulation |
| (VI) Personalized and multidisciplinary team approaches are necessary for management |
| (VII) An appropriate referral to the specialist may be needed for diagnosis and management of these comorbid conditions |
OMD, oromandibular dystonia; TMD, temporomandibular disorders.
Acknowledgments
Funding: This work was supported by
Footnote
Peer Review File: Available at https://joma.amegroups.com/article/view/10.21037/joma-23-34/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://joma.amegroups.com/article/view/10.21037/joma-23-34/coif). L.Y. received support from Orofacial Rehabilitation and Craniofacial Biology Research Fund (No. 25084) and grants from University of Minnesota. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Teemul TA, Patel R, Kanatas A, et al. Management of oromandibular dystonia with botulinum A toxin: a series of cases. Br J Oral Maxillofac Surg 2016;54:1080-4. [Crossref] [PubMed]
- Sude A, Nixdorf DR. Prevalence and clinical characteristics of patients with oromandibular dystonia seen in the orofacial pain clinic: a retrospective study. Oral Surg Oral Med Oral Pathol Oral Radiol 2020;130:169-74. [Crossref] [PubMed]
- Kalita J, Misra UK, Pradhan PK. Oromandibular dystonia in encephalitis. J Neurol Sci 2011;304:107-10. [Crossref] [PubMed]
- Lee KH. Oromandibular dystonia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104:491-6. [Crossref] [PubMed]
- Sankhla C, Lai EC, Jankovic J. Peripherally induced oromandibular dystonia. J Neurol Neurosurg Psychiatry 1998;65:722-8. [Crossref] [PubMed]
- Manzo N, Ginatempo F, Belvisi D, et al. Pathophysiological mechanisms of oromandibular dystonia. Clin Neurophysiol 2022;134:73-80. [Crossref] [PubMed]
- Ameer MA, Bhatti D. Chemodenervation for Oromandibular Dystonia Utilizing Botulinum Toxins. Cureus 2021;13:e18425. [Crossref] [PubMed]
- Bhidayasiri R, Maytharakcheep S, Truong DD. Patient selection and injection techniques for botulinum neurotoxin in oromandibular dystonia. Clin Park Relat Disord 2022;7:100160. [Crossref] [PubMed]
- Yoshida K. Botulinum Neurotoxin Injection for the Treatment of Recurrent Temporomandibular Joint Dislocation with and without Neurogenic Muscular Hyperactivity. Toxins (Basel) 2018;10:174. [Crossref] [PubMed]
- Yoshida K. Botulinum Toxin Therapy for Oromandibular Dystonia and Other Movement Disorders in the Stomatognathic System. Toxins (Basel) 2022;14:282. [Crossref] [PubMed]
- Tan EK, Jankovic J. Tardive and idiopathic oromandibular dystonia: a clinical comparison. J Neurol Neurosurg Psychiatry 2000;68:186-90. [Crossref] [PubMed]
- Shrivastava M, Tian G, Ye L. Rare diseases with temporomandibular joint manifestations: a systematic review. Rare Dis Orphan Drugs J 2023;2:24. [Crossref]
- Shrivastava M, Battaglino R, Ye L. A comprehensive review on biomarkers associated with painful temporomandibular disorders. Int J Oral Sci 2021;13:23. [Crossref] [PubMed]
- Handa S, Shaefer JR, Keith DA. Oromandibular dystonia and temporomandibular disorders. J Am Dent Assoc 2022;153:899-906. [Crossref] [PubMed]
- Merz RI, Deakin J, Hawthorne MR. Oromandibular dystonia questionnaire (OMDQ-25): a valid and reliable instrument for measuring health-related quality of life. Clin Otolaryngol 2010;35:390-6. [Crossref] [PubMed]
- Yoshida K. Development and Validation of a Disease-Specific Oromandibular Dystonia Rating Scale (OMDRS). Front Neurol 2020;11:583177. [Crossref] [PubMed]
- Yoshida K. Oromandibular dystonia screening questionnaire for differential diagnosis. Clin Oral Investig 2019;23:405-11. [Crossref] [PubMed]
- Fung VS, Jinnah HA, Bhatia K, et al. Assessment of patients with isolated or combined dystonia: an update on dystonia syndromes. Mov Disord 2013;28:889-98. [Crossref] [PubMed]
- Jinnah HA, Factor SA. Diagnosis and treatment of dystonia. Neurol Clin 2015;33:77-100. [Crossref] [PubMed]
- Jinnah HA. Medical and Surgical Treatments for Dystonia. Neurol Clin 2020;38:325-48. [Crossref] [PubMed]
- Martino D, Berardelli A, Abbruzzese G, et al. Age at onset and symptom spread in primary adult-onset blepharospasm and cervical dystonia. Mov Disord 2012;27:1447-50. [Crossref] [PubMed]
- Ludlow CL, Domangue R, Sharma D, et al. Consensus-Based Attributes for Identifying Patients With Spasmodic Dysphonia and Other Voice Disorders. JAMA Otolaryngol Head Neck Surg 2018;144:657-65. [Crossref] [PubMed]
- Powis Z, Towne MC, Hagman KDF, et al. Clinical diagnostic exome sequencing in dystonia: Genetic testing challenges for complex conditions. Clin Genet 2020;97:305-11. [Crossref] [PubMed]
- Shrivastava M, Ye L. Toward an enhanced understanding of relationship between insomnia and painful temporomandibular disorder: An integrative review. Essent Dent 2023;2:122-34. [Crossref]
- Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group†. J Oral Facial Pain Headache 2014;28:6-27. [Crossref] [PubMed]
- Shrivastava M, Ye L. A Review on Autophagy in Orofacial Neuropathic Pain. Cells 2022;11:3842. [Crossref] [PubMed]
- Hassell TJW, Charles D. Treatment of Blepharospasm and Oromandibular Dystonia with Botulinum Toxins. Toxins (Basel) 2020;12:269. [Crossref] [PubMed]
- Dimitroulis G. Management of temporomandibular joint disorders: A surgeon's perspective. Aust Dent J 2018;63:S79-90. [Crossref] [PubMed]
- Maestre-Ferrín L, Burguera JA, Peñarrocha-Diago M, et al. Oromandibular dystonia: a dental approach. Med Oral Patol Oral Cir Bucal 2010;15:e25-7. [PubMed]
- Saraf U, Chandarana M, Divya KP, et al. Oromandibular Dystonia - A Systematic Review. Ann Indian Acad Neurol 2022;25:26-34. [Crossref] [PubMed]
- Ma H, Qu J, Ye L, et al. Blepharospasm, Oromandibular Dystonia, and Meige Syndrome: Clinical and Genetic Update. Front Neurol 2021;12:630221. [Crossref] [PubMed]
- Brüggemann N, Kühn A, Schneider SA, et al. Short- and long-term outcome of chronic pallidal neurostimulation in monogenic isolated dystonia. Neurology 2015;84:895-903. [Crossref] [PubMed]
- Spiegel LL, Ostrem JL, Bledsoe IO. FDA Approvals and Consensus Guidelines for Botulinum Toxins in the Treatment of Dystonia. Toxins (Basel) 2020;12:332. [Crossref] [PubMed]
- Delcanho R, Val M, Guarda Nardini L, et al. Botulinum Toxin for Treating Temporomandibular Disorders: What is the Evidence? J Oral Facial Pain Headache 2022;36:6-20. [Crossref] [PubMed]
- Patil S, Willett O, Thompkins T, et al. Botulinum Toxin: Pharmacology and Therapeutic Roles in Pain States. Curr Pain Headache Rep 2016;20:15. [Crossref] [PubMed]
- Rivera Día RC, Lotero MAA, Suarez MVA, et al. Botulinum toxin for the treatment of chronic pain. Review of the evidence. Colombian Journal of Anesthesiology 2014;42:205-13. [Crossref]
- Dadgardoust PD, Rosales RL, Asuncion RM, et al. Botulinum neurotoxin a therapy efficacy and safety for oromandibular dystonia: a meta-analysis. J Neural Transm (Vienna) 2019;126:141-8. [Crossref] [PubMed]
- Hallett M, Albanese A, Dressler D, et al. Evidence-based review and assessment of botulinum neurotoxin for the treatment of movement disorders. Toxicon 2013;67:94-114. [Crossref] [PubMed]
- Dressler D, Altavista MC, Altenmueller E, et al. Consensus guidelines for botulinum toxin therapy: general algorithms and dosing tables for dystonia and spasticity. J Neural Transm (Vienna) 2021;128:321-35. [Crossref] [PubMed]
- Bakke M, Dalager T, Møller E. What clinical strategies are applied for botulinum toxin injection in the oromandibular region? IntechOpen 2016. doi:
10.5772/64271 . - Bhidayasiri R, Cardoso F, Truong DD. Botulinum toxin in blepharospasm and oromandibular dystonia: comparing different botulinum toxin preparations. Eur J Neurol 2006;13:21-9. [Crossref] [PubMed]
- Mendes RA, Upton LG. Management of dystonia of the lateral pterygoid muscle with botulinum toxin A. Br J Oral Maxillofac Surg 2009;47:481-3. [Crossref] [PubMed]
- Karp BI, Alter K. Botulinum Toxin Treatment of Blepharospasm, Orofacial/Oromandibular Dystonia, and Hemifacial Spasm. Semin Neurol 2016;36:84-91. [Crossref] [PubMed]
- Tan EK, Jankovic J. Botulinum toxin A in patients with oromandibular dystonia: long-term follow-up. Neurology 1999;53:2102-7. [Crossref] [PubMed]
- Charnukha T, Likhachev S, Navosha S. Experience of botulinum toxin in treatment of oromandibular dystonia. Toxicon 2016;123:S15-6. [Crossref]
- Sinclair CF, Gurey LE, Blitzer A. Oromandibular dystonia: long-term management with botulinum toxin. The Laryngoscope. 2013;123:3078-83. [Crossref] [PubMed]
- Yu GLT, Rosales RL. Treatment of oromandibular dystonia using botulinum toxin injections–Case series and illustrative muscle targeting. Basal Ganglia 2018;13:7-16. [Crossref]
Cite this article as: Shrivastava M, Ye L. Oromandibular dystonia and temporomandibular disorders—a review on diagnosis and management. J Oral Maxillofac Anesth 2024;3:13.



